Drug Detail:Quviviq (Daridorexant)
Drug Class: Miscellaneous anxiolytics, sedatives and hypnotics
Highlights of Prescribing Information
QUVIVIQ (daridorexant) tablets, for oral use, CIV
Initial U.S. Approval: 2022
Indications and Usage for Quviviq
QUVIVIQ is an orexin receptor antagonist indicated for the treatment of adult patients with insomnia, characterized by difficulties with sleep onset and/or sleep maintenance. (1)
Quviviq Dosage and Administration
- The recommended dosage is 25 mg to 50 mg once per night, taken orally within 30 minutes before going to bed, with at least 7 hours remaining prior to planned awakening. (2.1)
- Time to sleep onset may be delayed if taken with or soon after a meal. (2.1)
- Hepatic Impairment: (2.3)
- Moderate hepatic impairment: Maximum recommended dosage is 25 mg no more than once per night.
- Severe hepatic impairment: Not recommended.
Dosage Forms and Strengths
Tablets: 25 mg, 50 mg. (3)
Contraindications
QUVIVIQ is contraindicated in patients with narcolepsy. (4)
Warnings and Precautions
- CNS-Depressant Effects and Daytime Impairment: Impairs alertness and motor coordination including morning impairment. Risk increases when used with other central nervous system (CNS) depressants. For patients taking QUVIVIQ, caution against next-day driving and other activities requiring complete mental alertness. (5.1)
- Worsening of Depression/Suicidal Ideation: Worsening of depression or suicidal thinking may occur. (5.2)
- Sleep Paralysis, Hypnagogic/Hypnopompic Hallucinations, and Cataplexy-like Symptoms: May occur with use of QUVIVIQ. (5.3)
- Complex Sleep Behaviors: Behaviors including sleepwalking, sleep driving, and engaging in other activities while not fully awake may occur. Discontinue immediately if complex sleep behavior occurs. (5.4)
- Compromised Respiratory Function: Effect on respiratory function should be considered. (5.5, 8.7)
- Need to Evaluate for Co-morbid Diagnoses: Reevaluate if insomnia persists after 7 to 10 days. (5.6)
Adverse Reactions/Side Effects
The most common adverse reactions (reported in ≥ 5% of patients treated with QUVIVIQ and at an incidence ≥ than placebo) were headache and somnolence or fatigue. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Idorsia Pharmaceuticals Ltd at toll-free phone 1-833-400-9611 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Strong CYP3A4 inhibitors: Avoid concomitant use. (2.2, 7.1)
- Moderate CYP3A4 inhibitors: Maximum recommended dose is 25 mg. (2.2, 7.1)
- Moderate or Strong CYP3A4 inducers: Avoid concomitant use. (7.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2023
Related/similar drugs
Belsomra, Dayvigo, lorazepam, melatonin, zolpidem, diphenhydramine, AtivanFull Prescribing Information
1. Indications and Usage for Quviviq
QUVIVIQ is indicated for the treatment of adult patients with insomnia, characterized by difficulties with sleep onset and/or sleep maintenance [see Clinical Studies (14.1)].
2. Quviviq Dosage and Administration
2.1 Recommended Dosage
The recommended dosage range is 25 mg to 50 mg of QUVIVIQ taken orally no more than once per night within 30 minutes of going to bed (with at least 7 hours remaining prior to planned awakening).
Time to sleep onset may be delayed if taken with or soon after a meal [see Clinical Pharmacology (12.3)].
2.3 Dosage Recommendations for Patients with Hepatic Impairment
The maximum recommended dosage in patients with moderate hepatic impairment (Child-Pugh score 7–9) is 25 mg of QUVIVIQ no more than once per night [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
QUVIVIQ is not recommended in patients with severe hepatic impairment (Child-Pugh score ≥ 10) [see Use in Specific Populations (8.6)].
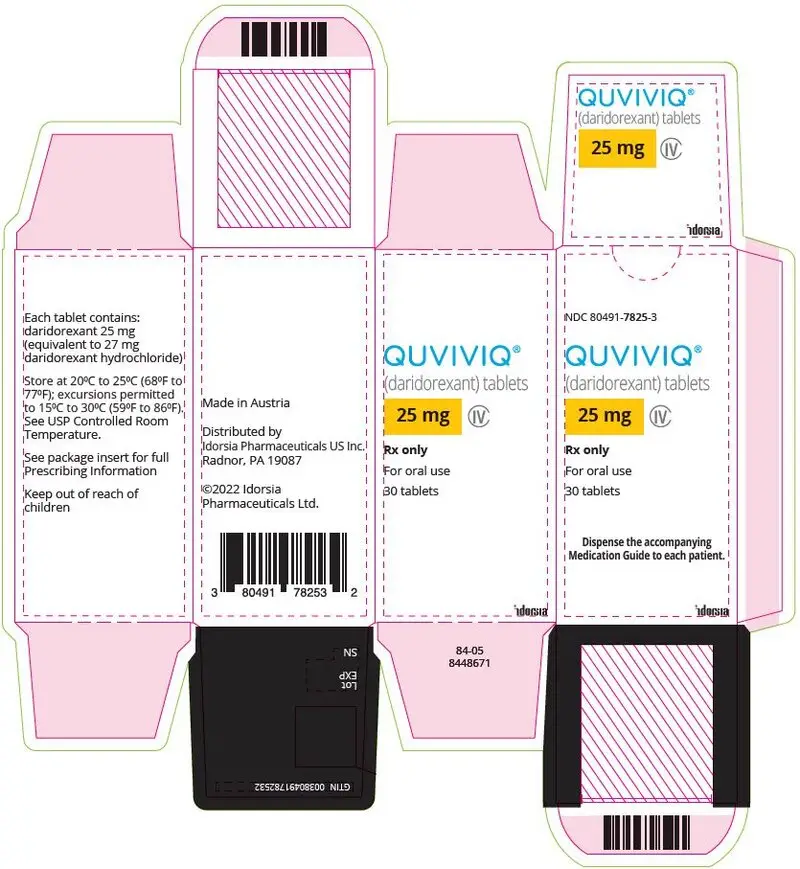
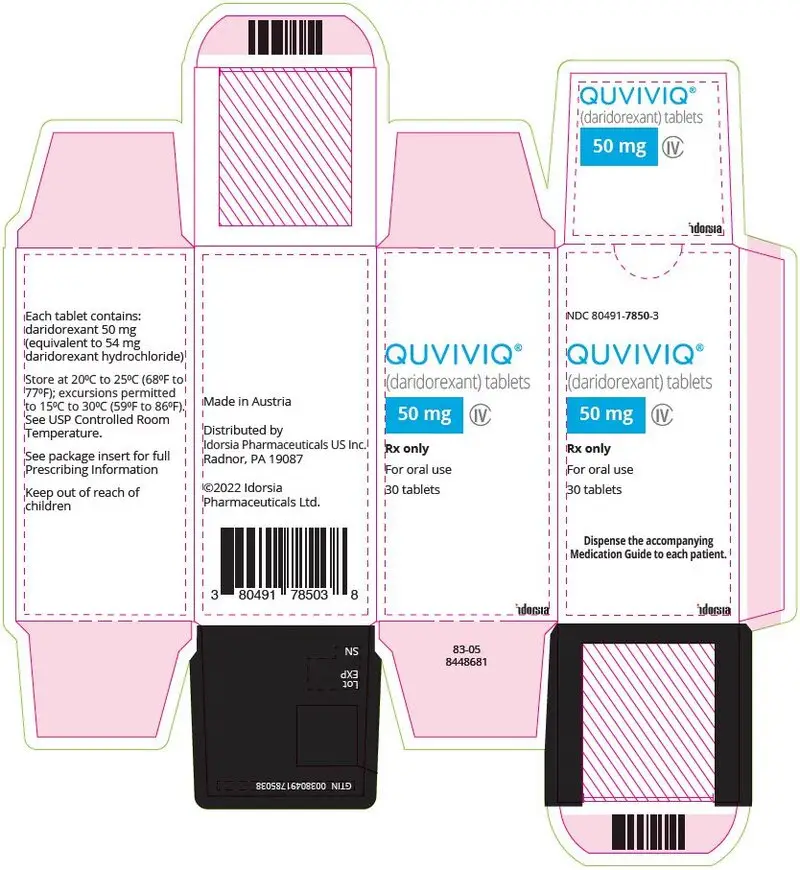
3. Dosage Forms and Strengths
QUVIVIQ (daridorexant) tablets are available as:
25 mg: light purple, arc-triangle shaped, film-coated tablet debossed with "25" on one side and "i" (Idorsia logo) on the other side, containing 25 mg daridorexant.
50 mg: light orange, arc-triangle shaped, film-coated tablet debossed with "50" on one side and "i" (Idorsia logo) on the other side, containing 50 mg daridorexant.
5. Warnings and Precautions
5.1 CNS-Depressant Effects and Daytime Impairment
QUVIVIQ is a central nervous system (CNS) depressant that can impair daytime wakefulness even when used as prescribed. CNS-depressant effects may persist in some patients for up to several days after discontinuing QUVIVIQ. Prescribers should advise patients about the potential for next-day somnolence.
Driving ability was impaired in some subjects taking QUVIVIQ 50 mg [see Clinical Studies (14.2)]. The risk of daytime impairment is increased if QUVIVIQ is taken with less than a full night of sleep remaining or if a higher than recommended dose is taken [see Dosage and Administration (2.1)]. If QUVIVIQ is taken in these circumstances, caution patients against driving and other activities requiring complete mental alertness.
Co-administration with other CNS depressants (e.g., benzodiazepines, opioids, tricyclic antidepressants, alcohol) increases the risk of CNS depression, which can cause daytime impairment. Dosage adjustments of QUVIVIQ and of concomitant CNS depressants may be necessary when administered together because of potentially additive effects. The use of QUVIVIQ with other drugs to treat insomnia is not recommended. Advise patients not to consume alcohol in combination with QUVIVIQ because co-administration of QUVIVIQ with alcohol resulted in additive effects on psychomotor performance [see Drug Interactions (7.1)].
Because QUVIVIQ can cause drowsiness, patients, particularly the elderly, are at a higher risk of falls.
5.2 Worsening of Depression/Suicidal Ideation
Patients with psychiatric disorders, including insomnia, are at increased risk of suicide. In primarily depressed patients treated with hypnotics, worsening of depression and suicidal thoughts and actions (including completed suicides) have been reported. As with other hypnotics, QUVIVIQ should be administered with caution in patients exhibiting symptoms of depression. Monitoring of suicide risk and protective measures may be required.
5.3 Sleep Paralysis, Hypnagogic/Hypnopompic Hallucinations, and Cataplexy-like Symptoms
Sleep paralysis, an inability to move or speak for up to several minutes during sleep-wake transitions, and hypnagogic/hypnopompic hallucinations, including vivid and disturbing perceptions, can occur with the use of QUVIVIQ [see Adverse Reactions (6.1)]. Prescribers should explain the nature of these events to patients when prescribing QUVIVIQ.
Symptoms similar to mild cataplexy have been reported with orexin receptor antagonists. Such symptoms can include periods of leg weakness lasting from seconds to a few minutes, can occur either at night or during the day, and may not be associated with an identified triggering event (e.g., laughter or surprise)
5.4 Complex Sleep Behaviors
Complex sleep behaviors, including sleepwalking, sleep driving, and engaging in other activities while not fully awake (e.g., preparing and eating food, making phone calls, having sex), have been reported to occur with the use of hypnotics, including orexin receptor antagonists such as QUVIVIQ. These events can occur in hypnotic-naïve as well as in hypnotic-experienced persons. Patients usually do not remember these events. Complex sleep behaviors may occur following the first or any subsequent use of hypnotics, such as QUVIVIQ, with or without the concomitant use of alcohol and other CNS depressants [see Drug Interactions (7.1)]. Discontinue QUVIVIQ immediately if a patient experiences a complex sleep behavior.
5.5 Patients with Compromised Respiratory Function
The effects of QUVIVIQ on respiratory function should be considered if prescribed to patients with compromised respiratory function. QUVIVIQ has not been studied in patients with moderate OSA requiring CPAP or severe OSA. QUVIVIQ has not been studied in patients with severe COPD [see Use in Specific Populations (8.7)].
5.6 Need to Evaluate for Co-morbid Diagnoses
Because sleep disturbances may be the presenting manifestation of a medical and/or psychiatric disorder, treatment of insomnia should be initiated only after careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia or the emergence of new cognitive or behavioral abnormalities may be the result of an unrecognized underlying psychiatric or medical disorder and can emerge during the course of treatment with sleep-promoting drugs such as QUVIVIQ.
6. Adverse Reactions/Side Effects
The following are discussed in detail in other sections of the labeling:
- CNS-Depressant Effects and Daytime Impairment [see Warnings and Precautions (5.1)]
- Worsening of Depression/Suicidal Ideation [see Warnings and Precautions (5.2)]
- Sleep Paralysis, Hypnagogic/Hypnopompic Hallucinations, and Cataplexy-like Symptoms [see Warnings and Precautions (5.3)]
- Complex Sleep Behaviors [see Warnings and Precautions (5.4)]
- Patients with Compromised Respiratory Function [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
The safety of QUVIVIQ was evaluated in three placebo-controlled clinical studies (two 3-month studies of identical design [Study 1 and Study 2], and a 9-month extension study [Study 3]). Study 1 evaluated 50 mg and 25 mg doses of QUVIVIQ, while Study 2 evaluated a 25 mg dose and a 10 mg dose of QUVIVIQ. The 10 mg dose is not an approved dose. A total of 1232 patients (including approximately 40% elderly patients [≥ 65 years old]), received QUVIVIQ 50 mg (N = 308); 25 mg (N = 618); or 10 mg (an unapproved dose) (N = 306). A total of 576 patients were treated with QUVIVIQ for at least 6 months and 331 for at least 12 months.
Most Common Adverse Reactions
The most common reported adverse reaction (in at least 5% of patients and greater than placebo) during double-blind treatment in Study 1 was headache.
Table 1 shows adverse reactions that occurred in at least 2% of patients treated with QUVIVIQ and more frequently than in patients who received placebo in Study 1.
| QUVIVIQ | QUVIVIQ | Placebo | |
|---|---|---|---|
| 25 mg | 50 mg | ||
| (N=310) | (N=308) | (N=309) | |
| % | % | % | |
|
|||
| Nervous System Disorders | |||
| Headache* | 6 | 7 | 5 |
| Somnolence or fatigue* | 6 | 5 | 4 |
| Dizziness* | 2 | 3 | 2 |
| Gastro-intestinal disorders | |||
| Nausea* | 0 | 3 | 2 |
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of QUVIVIQ. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Psychiatric disorders: Abnormal dreams or nightmares
Immune system disorders: Hypersensitivity (such as rash, urticaria)
7. Drug Interactions
7.1 Drugs Having Clinically Important Interactions with QUVIVIQ
| Strong or Moderate CYP3A4 Inhibitors | |
| Clinical Implications: | Concomitant use with a strong or moderate CYP3A4 inhibitor increases exposure to daridorexant [see Clinical Pharmacology (12.3)], which may increase the risk of QUVIVIQ adverse reactions. |
| Prevention or Management: | The recommended dose of QUVIVIQ is 25 mg when used with a moderate CYP3A4 inhibitor [see Dosage and Administration (2.2)]. Concomitant use of QUVIVIQ with a strong inhibitor of CYP3A4 is not recommended [see Dosage and Administration (2.2)]. |
| Strong and Moderate CYP3A4 Inducers | |
| Clinical Implications: | Concomitant use with a strong or moderate CYP3A4 inducer decreases exposure to daridorexant [see Clinical Pharmacology (12.3)], which may reduce the efficacy of QUVIVIQ. |
| Prevention or Management: | Concomitant use of QUVIVIQ with a strong or moderate inducer of CYP3A4 is not recommended [see Dosage and Administration (2.2)]. |
| Alcohol and Other CNS Depressants | |
| Clinical Implications: | Concomitant use of alcohol or other CNS depressants with QUVIVIQ may lead to additive impairment of psychomotor performance and risk of CNS depression [see Clinical Pharmacology (12.2)]. |
| Prevention or Management: | Avoid alcohol consumption with QUVIVIQ [see Warnings and Precautions (5.1)]. Use with caution in patients receiving CNS depressants. Consider dose adjustment of QUVIVIQ and/or the CNS depressant(s) if used concomitantly [see Warnings and Precautions (5.1)]. |
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of QUVIVIQ have not been established in pediatric patients.
8.5 Geriatric Use
No dose adjustment is required in patients over the age of 65 years.
Of the total number of subjects in the clinical studies of QUVIVIQ with insomnia (N = 1854), approximately 39% (N = 727) were ≥ 65 years and 5.9% (N = 110) were ≥ 75 years. The likelihood of somnolence and fatigue increased with patient age.
Because QUVIVIQ can increase somnolence and drowsiness, patients, particularly the elderly, are at higher risk of falls [see Warnings and Precautions (5.1)].
8.6 Hepatic Impairment
QUVIVIQ has not been studied in patients with severe hepatic impairment (Child-Pugh score ≥ 10). Use in this population is not recommended [see Clinical Pharmacology (12.3)].
Reduce the dose of QUVIVIQ in patients with moderate hepatic impairment (Child-Pugh score 7–9) [see Dosage and Administration (2.3)]. Moderate hepatic impairment may increase daridorexant systemic exposure to a clinically relevant extent [see Clinical Pharmacology (12.3)], which may increase the frequency or severity of adverse reactions.
9. Drug Abuse and Dependence
9.2 Abuse
Drug abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. The abuse potential of daridorexant was evaluated in preclinical models, recreational sedative drug users, and insomnia subjects.
In a human abuse potential study conducted in 63 recreational sedative drug users, the effect of single-dose administration of QUVIVIQ [50 mg, 100 mg (two times the maximum recommended dose), and 150 mg (three times the maximum recommended dose)], zolpidem (30 mg), suvorexant (150 mg), and placebo on subjective rating of "drug liking" was evaluated. At the dose of 50 mg, QUVIVIQ showed significantly lower "drug liking" ratings than zolpidem (30 mg) and suvorexant (150 mg), but significantly higher than placebo. At doses of 100 mg (two times the maximum recommended dose) and 150 mg (three times the maximum recommended dose), QUVIVIQ showed similar "drug liking" ratings to zolpidem (30 mg) and suvorexant (150 mg).
In placebo-controlled Phase 3 clinical studies in which 1232 subjects with insomnia were treated with QUVIVIQ for up to 12 months, there were no reports indicative of abuse liability. Because individuals with a history of abuse of or addiction to alcohol or other drugs may be at increased risk for abuse of or addiction to QUVIVIQ, follow such patients carefully.
9.3 Dependence
Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms upon abrupt treatment discontinuation or a significant dose reduction of a drug.
In animal studies and clinical trials evaluating physical dependence, chronic administration of daridorexant did not produce withdrawal signs or symptoms upon drug discontinuation. This suggests that daridorexant does not produce physical dependence.
10. Overdosage
There is limited clinical experience with QUVIVIQ overdose. In clinical pharmacology studies, healthy subjects were administered single doses of up to 200 mg (4 times the maximum recommended dose) of QUVIVIQ. The following adverse reactions were observed: somnolence, muscle weakness, cataplexy-like symptoms, sleep paralysis, disturbance in attention, fatigue, headache, and constipation.
There is no specific antidote to an overdosage of QUVIVIQ. In the event of an overdose, general symptomatic and supportive medical care, along with immediate gastric lavage where appropriate, should be provided and patients should be carefully monitored. Dialysis is unlikely to be effective as daridorexant is highly protein bound. Consult a Certified Poison Control Center for the most up to date information on the management of overdosage (1-800-222-1222 or www.poison.org).
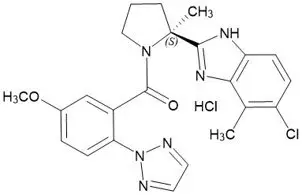
11. Quviviq Description
QUVIVIQ contains daridorexant, an orexin receptor antagonist, present as daridorexant hydrochloride salt. The chemical name of daridorexant hydrochloride is (S)-(2-(5-chloro-4-methyl-1H-benzo[d]imidazol-2-yl)-2-methylpyrrolidin-1-yl)(5-methoxy-2-(2H-1,2,3-triazol-2-yl)phenyl)methanone hydrochloride. The molecular formula is C23H23N6O2Cl * HCl. The molecular weight is 487.38 g/mol.
The structural formula is:
Daridorexant hydrochloride is a white to light yellowish powder that is very slightly soluble in water.
QUVIVIQ tablets are intended for oral administration. Each film-coated tablet contains daridorexant 25 mg or 50 mg, equivalent to 27 mg or 54 mg of daridorexant hydrochloride, respectively. The inactive ingredients are croscarmellose sodium, magnesium stearate, mannitol, microcrystalline cellulose, povidone, and silicon dioxide.
In addition, the film coating contains the following inactive ingredients: glycerin, hypromellose, iron oxide black, iron oxide red, microcrystalline cellulose, talc, titanium dioxide, and, in the 50 mg tablet only, iron oxide yellow.
12. Quviviq - Clinical Pharmacology
12.1 Mechanism of Action
The mechanism of action of daridorexant in the treatment of insomnia is presumed to be through antagonism of orexin receptors. The orexin neuropeptide signaling system plays a role in wakefulness. Blocking the binding of wake-promoting neuropeptides orexin A and orexin B to receptors OX1R and OX2R is thought to suppress wake drive.
12.2 Pharmacodynamics
Daridorexant binds to and inhibits the orexin receptors OX1R and OX2R (Ki = 0.47 and 0.93 nM, respectively).
12.3 Pharmacokinetics
Daridorexant plasma exposure is dose proportional from 25 mg to 50 mg. The daridorexant pharmacokinetic profile is similar following multiple-dose and single-dose administration with no accumulation.
Specific Populations
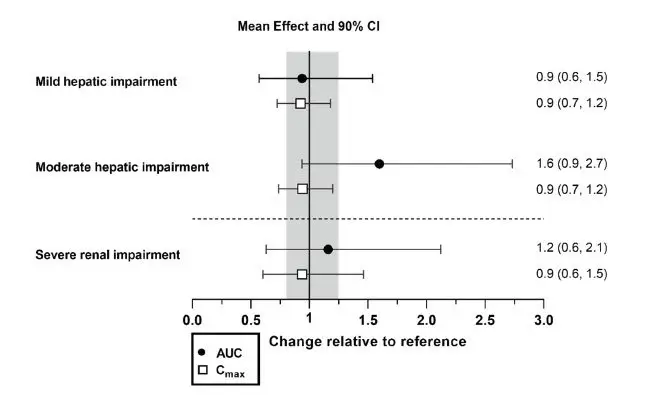
Age, sex, race (White, Black, Asian), body size, and mild to severe renal impairment (Cockcroft-Gault < 30 mL/min, not on dialysis) did not have a clinically significant effect on the pharmacokinetics of daridorexant. The effect of severe hepatic impairment (Child-Pugh score ≥ 10) on the pharmacokinetics of daridorexant has not been studied.
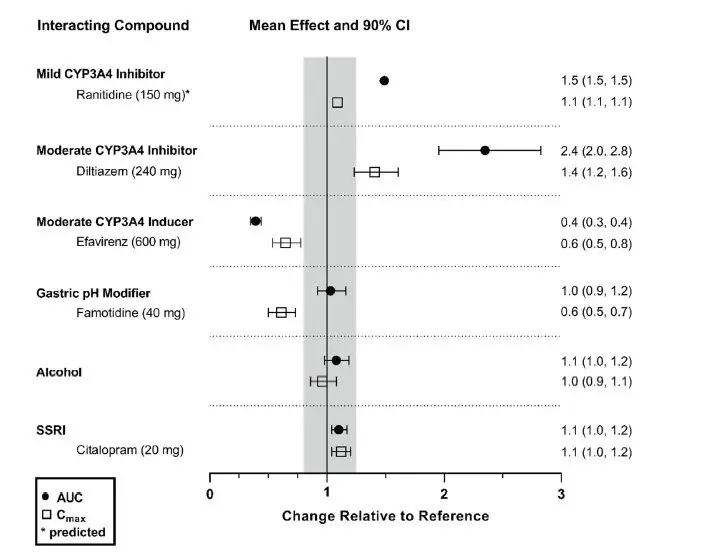
Effects of hepatic impairment and renal impairment on the exposure to daridorexant are summarized in Figure 1.
Figure 1 Effects of hepatic impairment and renal impairment on daridorexant PK
| Daridorexant dose: 25 mg. Data are GMRs and 90% CIs. Hepatic impairment PK variables are based on the unbound fraction of daridorexant. Reference = matched healthy subjects. AUC = area under the plasma concentration-time curve from zero to infinity; CI = confidence interval; Cmax = maximum plasma concentration; GMR = geometric mean ratio; PK = pharmacokinetics. |
 |
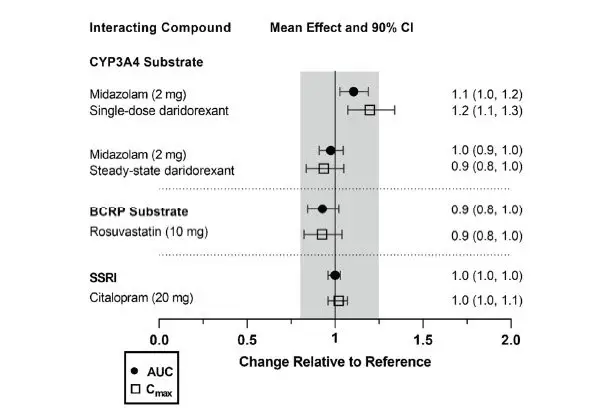
Drug Interaction Studies
The effects of other compounds on the exposure to daridorexant are summarized in Figure 2. The effects of daridorexant on the exposure to other compounds are summarized in Figure 3.
Effect of Other Compounds on QUVIVIQ
Figure 2 Effect of co-administered compounds on the PK of daridorexant
| Daridorexant 50 mg was administered with interacting drugs, except with diltiazem (25 mg daridorexant). Interacting drugs were administered in multiple-dose fashion, except famotidine (single dose) and alcohol (5 h infusion at 0.6 g/L). Based on PBPK analysis: Concomitant use of itraconazole (a strong CYP3A4 inhibitor) increased daridorexant AUC by more than 400%. Concomitant use of rifampin (a strong CYP3A4 inducer) decreased daridorexant AUC by more than 50%. Data are GMRs and 90% CIs. Some 90% CIs are too narrow to be shown. AUC = area under the plasma concentration-time curve; CI = confidence interval; Cmax = maximum plasma concentration; GMR = geometric mean ratio; SSRI = selective serotonin reuptake inhibitor. |
 |
Effect of QUVIVIQ on Other Compounds
Figure 3 Effect of daridorexant on the PK of other compounds
| Single- and multiple-dose (on Day 4) daridorexant 25 mg administered in studies with midazolam and rosuvastatin (only multiple dose). Single-dose daridorexant 50 mg administered in study with citalopram (citalopram at steady state). Data are GMRs and 90% CIs. AUC = area under the plasma concentration-time curve. CI = confidence interval. Cmax = maximum plasma concentration. GMR = geometric mean ratio. SSRI = selective serotonin reuptake inhibitor. |
 |
13. Nonclinical Toxicology
13.2 Animal Toxicology and/or Pharmacology
In dogs, daily oral administration of daridorexant at ≥ 30 mg/kg/day resulted in behavior characteristic of cataplexy when presented with positive stimulation. The no-observed-effect level (NOEL) for cataplexy is 20 mg/kg/day, which is approximately 3 times the MRHD of 50 mg, based on Cmax and AUC.
14. Clinical Studies
14.1 Controlled Clinical Studies
The efficacy of QUVIVIQ was evaluated in two multicenter, randomized, double-blind, placebo-controlled, parallel-group studies, Study 1 (NCT03545191) and Study 2 (NCT03575104).
A total of 1854 patients with Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5®) insomnia were randomized to receive QUVIVIQ or placebo once daily, in the evening, for 3 months. Study 1 randomized 930 subjects to QUVIVIQ 50 mg (N = 310), 25 mg (N = 310) or placebo (N = 310). Study 2 randomized 924 subjects to QUVIVIQ 25 mg (N = 309), 10 mg (N = 307), or placebo (N = 308). The 10 mg dose is not an approved dose.
At the end of the 3-month treatment period, both studies included a 7-day placebo run-out period, after which patients could enter a 9-month, double-blind, placebo-controlled extension study (Study 3, NCT03679884).
In Study 1, patients had a mean age of 55.4 years (range 18 to 88 years), with 39.1% of subjects ≥ 65 years of age, including 5.8% ≥ 75 years of age. Patients were identified as female or male and by US census-based racial and ethnic categories. The percentages of patients in the respective categories were: female sex (67.1%), White (90%), Black or African American (8%), Asian (1.0%), or Other race (< 1%).
In Study 2, patients had a mean age of 56.7 years (range 19 to 85 years), with 39.3% of subjects ≥ 65 years of age, including 6.1% ≥ 75 years of age. Patients were identified as female or male and by US census-based racial and ethnic categories. The percentages of patients in the respective categories were: female sex (69.0%), White (88%), Black or African American (8%), Asian (4%), or Other race (< 1%).
Primary efficacy endpoints for both studies were the change from baseline to Month 1 and Month 3 in Latency to Persistent Sleep (LPS) and Wake After Sleep Onset (WASO), measured objectively by polysomnography in a sleep laboratory. LPS is a measure of sleep induction and WASO is a measure of sleep maintenance.
Secondary endpoint included in the statistical testing hierarchy with Type 1 error control was patient-reported Total Sleep Time (sTST), evaluated every morning at home using a validated Sleep Diary Questionnaire (SDQ).
In Study 1, doses of 25 and 50 mg QUVIVIQ showed a statistically significant improvement vs placebo on polysomnography (LPS, WASO) and self-reported total sleep (sTST), at Month 1 and Month 3 (Table 3).
In Study 2, QUVIVIQ 25 mg showed a statistically significant improvement vs placebo on WASO and sTST at Month 1 and Month 3 (Table 4). QUVIVIQ 10 mg did not show a statistically significant improvement on LPS, WASO, or sTST at Month 1 or Month 3.
The efficacy of QUVIVIQ was similar across subgroups based on age, sex, race, and region.
| Treatment group/dose (N) | Baseline | Month 1 | Month 3 | ||||
|---|---|---|---|---|---|---|---|
| Change from baseline | Difference to placebo | Change from baseline | Difference to placebo | ||||
| mean (SD) | mean (SD) | LSM (95%CL) | LSM (95%CL) | mean (SD) | LSM (95%CL) | LSM (95%CL) |
|
| CL = confidence limit; LPS = latency to persistent sleep; LSM = least squares mean; PSG = polysomnography; SD = standard deviation; sTST = subjective total sleep time; WASO = wake after sleep onset. | |||||||
|
|||||||
| WASO (wake after sleep onset, min): sleep maintenance, assessed by PSG | |||||||
| 50 mg (310) | 95 (38) | 65 (35) | -29 [-33, -25] | -23*
[-28, -18] | 65 (39) | -29 [-33, -25] | -18*
[-24, -13] |
| 25 mg (310) | 98 (39) | 77 (42) | -18 [-22, -15] | -12*
[-17, -7] | 73 (40) | -23 [-27, -19] | -12*
[-17, -6] |
| placebo (310) | 103 (41) | 92 (42) | -6 [-10, -2] | 87 (43) | -11 [-15, -7] | ||
| LPS (latency to persistent sleep, min): sleep onset, assessed by PSG | |||||||
| 50 mg (310) | 64 (37) | 34 (27) | -31 [-35, -28] | -11*
[-16, -7] | 30 (23) | -35 [-38, -31] | -12*
[-16, -7] |
| 25 mg (310) | 67 (39) | 38 (32) | -28 [-32, -25] | -8*
[-13, -4] | 36 (34) | -31 [-34, -27] | -8*
[-12, -3] |
| placebo (310) | 67 (40) | 46 (36) | -20 [-23, -17] | 43 (34) | -23 [-26, -20] | ||
| sTST (subjective total sleep time, min): patient-reported | |||||||
| 50 mg (310) | 313 (58) | 358 (74) | 44 [38, 49] | 22*
[14, 30] | 372 (79) | 58 [51, 64] | 20*
[11, 29] |
| 25 mg (310) | 310 (60) | 345 (66) | 34 [29, 40] | 13*
[5, 20] | 358 (72) | 48 [41, 54] | 10*
[1, 19] |
| placebo (310) | 316 (53) | 338 (65) | 22 [16, 27] | 354 (73) | 38 [31, 44] | ||
| Treatment group/dose (N) | Baseline | Month 1 | Month 3 | ||||
|---|---|---|---|---|---|---|---|
| Change from baseline | Difference to placebo | Change from baseline | Difference to placebo | ||||
| mean (SD) | mean (SD) | LSM (95%CL) | LSM (95%CL) | mean (SD) | LSM (95%CL) | LSM (95%CL) |
|
| CL = confidence limit; LPS = latency to persistent sleep; LSM = least squares mean; PSG = polysomnography; SD = standard deviation; sTST = subjective total sleep time; WASO = wake after sleep onset. | |||||||
|
|||||||
| WASO (wake after sleep onset, min): sleep maintenance, assessed by PSG | |||||||
| 25 mg (309) | 106 (49) | 80 (44) | -24 [-28, -20] | -12*
[-18, -6] | 80 (49) | -24 [-29, -19] | -10*
[-17, -4] |
| placebo (308) | 108 (49) | 93 (50) | -13 [-17, -8] | 91 (47) | -14 [-19, -9] | ||
| LPS (latency to persistent sleep, min): sleep onset, assessed by PSG | |||||||
| 25 mg (309) | 69 (41) | 42 (39) | -26 [-31, -22] | -6 [-12, -1] | 39 (37) | -29 [-33, -24] | -9 [-15, -3] |
| placebo (308) | 72 (46) | 50 (40) | -20 [-24, -16] | 49 (46) | -20 [-24, -15] | ||
| sTST (subjective total sleep time, min): patient-reported | |||||||
| 25 mg (309) | 308 (53) | 353 (67) | 44 [38, 49] | 16*
[8, 24] | 365 (70) | 56 [50, 63] | 19*
[10, 28] |
| placebo (308) | 308 (52) | 336 (63) | 28 [22, 33] | 347 (65) | 37 [31, 43] | ||
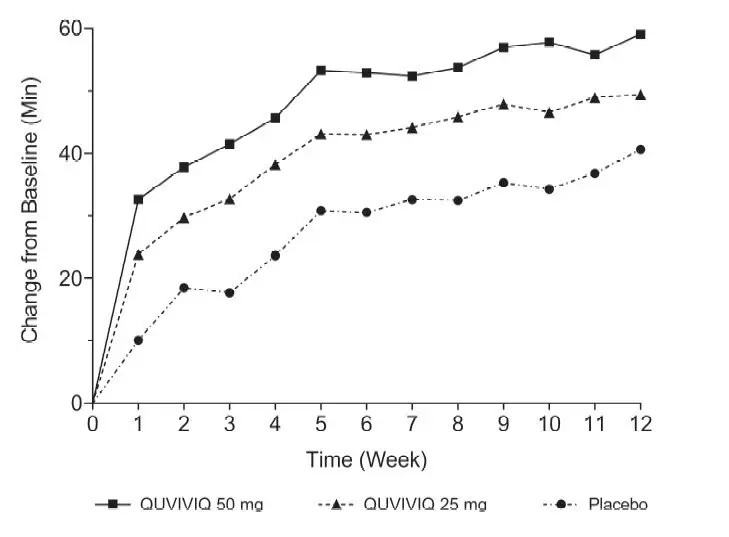
The effects of QUVIVIQ on LPS, WASO, and sTST were observed at Month 1 and were maintained through Month 3. The change from baseline of sTST by week in Study 1 is presented in Figure 4.
Figure 4 Change from Baseline of sTST by Week (Study 1)
16. How is Quviviq supplied
16.1 How Supplied
QUVIVIQ tablets are available as:
- 25 mg, light purple, arc-triangle shaped film-coated tablets debossed with "25" on one side, and "i" on the other side.
NDC 80491-7825-3, bottle of 30 with child-resistant closure - 50 mg: light orange, arc-triangle shaped film-coated tablets debossed with "50" on one side, and "i" on the other side.
NDC 80491-7850-3, bottle of 30 with child-resistant closure
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
| MEDICATION GUIDE QUVIVIQ® (cue-VIH-vick) (daridorexant) tablets, for oral use, CIV |
|
|---|---|
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Issued: 03/2023 |
| What is the most important information I should know about QUVIVIQ? QUVIVIQ may cause serious side effects, including:
|
|
What is QUVIVIQ?
|
|
| Who should not take QUVIVIQ? Do not take QUVIVIQ if you fall asleep often at unexpected times (narcolepsy). |
|
Before taking QUVIVIQ, tell your healthcare provider about all of your medical conditions, including if you:
Taking QUVIVIQ with certain medicines can cause serious side effects. QUVIVIQ may affect the way other medicines work and other medicines may affect the way QUVIVIQ works. Do not take QUVIVIQ with other medicines that can make you sleepy unless your healthcare provider tells you to. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
|
How should I take QUVIVIQ?
|
|
What should I avoid while taking QUVIVIQ?
|
|
| What are the possible side effects of QUVIVIQ?
QUVIVIQ may cause serious side effects, including:
These are not all of the possible side effects of QUVIVIQ. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store QUVIVIQ?
|
|
| General information about the safe and effective use of QUVIVIQ.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use QUVIVIQ for a condition for which it was not prescribed. Do not give QUVIVIQ to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about QUVIVIQ that is written for healthcare professionals. |
|
| What are the ingredients in QUVIVIQ? Active ingredient: daridorexant hydrochloride Inactive ingredients: croscarmellose sodium, magnesium stearate, mannitol, microcrystalline cellulose, povidone, and silicon dioxide. The tablet film coating contains: glycerin, hypromellose, iron oxide black, iron oxide red, microcrystalline cellulose, talc, titanium dioxide, and, in the 50 mg tablet only, iron oxide yellow. |
|
| Distributed by: Idorsia Pharmaceuticals US Inc. One Radnor Corporate Center, Suite 101 100 Matsonford Rd Radnor, PA 19087 For more information go to QUVIVIQ.com or call 1-833-400-9611. |
|
| QUVIVIQ
daridorexant tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| QUVIVIQ
daridorexant tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Idorsia Pharmaceuticals Ltd (480176487) |