Drug Detail:L-arginine (Medically reviewed)
Drug Class:
Warnings
There have been reports of overdosage of R-Gene 10 in pediatric patients leading to death. EXTREME CAUTION MUST BE EXERCISED WHEN INFUSING R-GENE 10 INTO PEDIATRIC PATIENTS. OVERDOSAGE OF R-GENE 10 IN PEDIATRIC PATIENTS CAN RESULT IN HYPERCHLOREMIC METABOLIC ACIDOSIS, CEREBRAL EDEMA, OR POSSIBLY DEATH.
Hypersensitivity reactions, including anaphylaxis have been reported. Appropriate medical support should be available during R-Gene 10 administration. If anaphylaxis or other serious hypersensitivity reaction occurs, R-Gene 10 should be discontinued and appropriate medical treatment initiated.
R-Gene 10 should always be administered by intravenous infusion because of its hypertonicity.
R-Gene 10 is a diagnostic aid and is not intended for therapeutic use.
R-Gene 10 Dosage and Administration
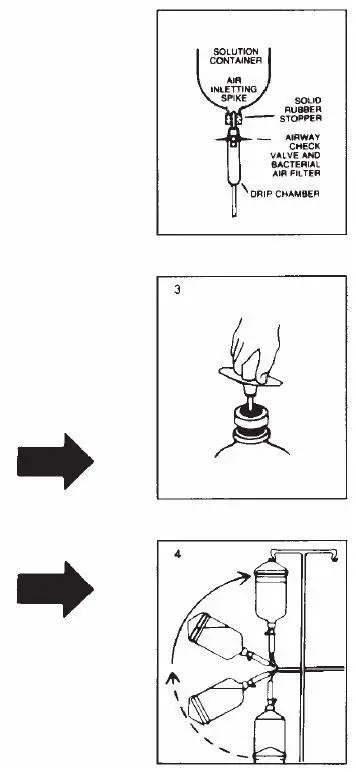
Directions for Use
R-Gene 10 is provided as a ready-to-use solution for patients weighing 60 kg (132 lbs) or more and should not be further diluted. For pediatric patients weighing 59 kg (130 lbs) or less a dose must be placed in a separate container. Follow the preparation instructions below.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
| R-GENE
arginine hydrochloride injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pharmacia & Upjohn Company LLC (618054084) |
| Registrant - Pfizer Inc (113480771) |