Drug Detail:Sarclisa (Isatuximab-irfc)
Drug Class: CD38 monoclonal antibodies
Highlights of Prescribing Information
SARCLISA® (isatuximab-irfc) injection, for intravenous use
Initial U.S. Approval: 2020
Recent Major Changes
| Dosage and Administration (2.2) | 7/2022 |
| Warnings and Precautions (5.2) | 7/2022 |
Indications and Usage for Sarclisa
SARCLISA is a CD38-directed cytolytic antibody indicated:
- in combination with pomalidomide and dexamethasone, for the treatment of adult patients with multiple myeloma who have received at least 2 prior therapies including lenalidomide and a proteasome inhibitor.
- in combination with carfilzomib and dexamethasone, for the treatment of adult patients with relapsed or refractory multiple myeloma who have received 1 to 3 prior lines of therapy. (1)
Sarclisa Dosage and Administration
- Premedicate with dexamethasone, acetaminophen, H2 antagonists, and diphenhydramine. (2.2)
- The recommended dose of SARCLISA is 10 mg/kg as an intravenous infusion every week for 4 weeks followed by every 2 weeks until disease progression or unacceptable toxicity. See full prescribing information for drugs used in combination and schedule. (2.1)
Dosage Forms and Strengths
Injection:
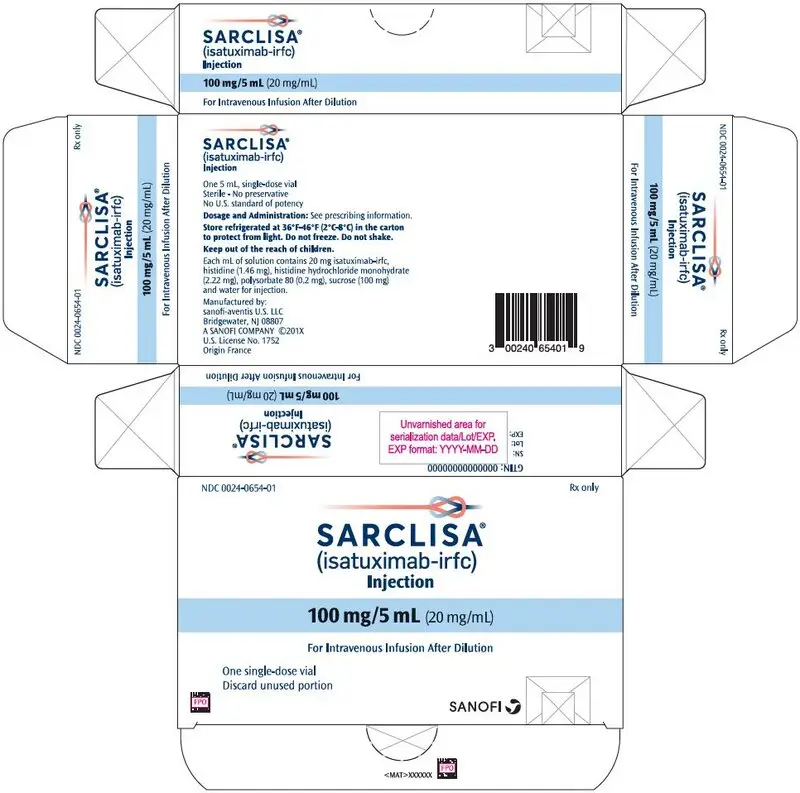
- 100 mg/5 mL (20 mg/mL) solution in single-dose vial (3)
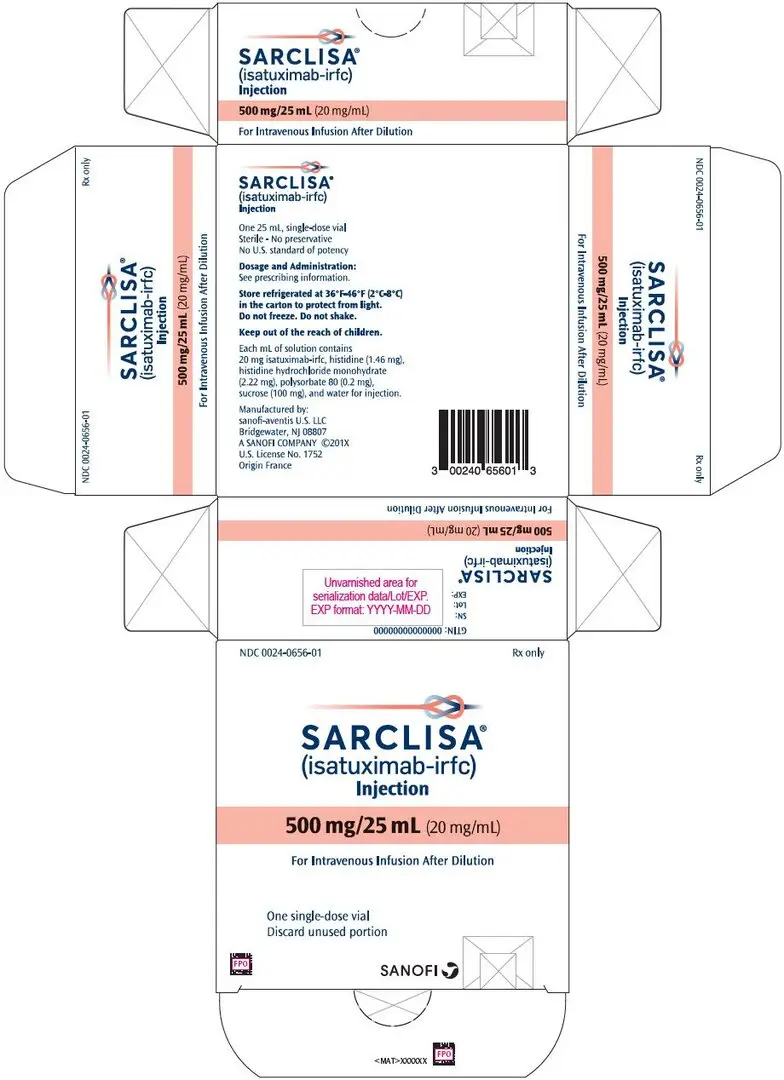
- 500 mg/25 mL (20 mg/mL) solution in single-dose vial (3)
Contraindications
Patients with severe hypersensitivity to isatuximab-irfc or to any of its excipients (4)
Warnings and Precautions
- Infusion-Related Reactions: In case of grade ≥2, interrupt SARCLISA and manage medically. Permanently discontinue for grade 4 infusion-related reactions or anaphylactic reaction. (5.1)
- Neutropenia: Monitor complete blood cell counts periodically during treatment. Monitor patients with neutropenia for signs of infection. SARCLISA dose delays and the use of colony-stimulating factor may be required to allow improvement of neutrophil count. (5.2)
- Second Primary Malignancies (SPM): Monitor patients for the development of second primary malignancies. (5.3)
- Laboratory Test Interference:
- Interference with Serological Testing (Indirect Antiglobulin Test): Type and screen patients prior to starting treatment. Inform blood banks that a patient has received SARCLISA. (5.4, 7.1)
- Interference with Serum Protein Electrophoresis and Immunofixation Tests: SARCLISA may interfere with the assays used to monitor M-protein, which may impact the determination of complete response. (5.4, 7.1)
- Embryo-Fetal Toxicity: Can cause fetal harm. (5.5)
Adverse Reactions/Side Effects
- In combination with pomalidomide and dexamethasone: The most common adverse reactions (≥20%) are upper respiratory tract infection, infusion-related reactions, pneumonia, and diarrhea. The most common hematology laboratory abnormalities (≥80%) are decreased hemoglobin, decreased neutrophils, decreased lymphocytes, and decreased platelets. (6.1)
- In combination with carfilzomib and dexamethasone: The most common adverse reactions (≥20%) are upper respiratory tract infection, infusion-related reactions, fatigue, hypertension, diarrhea, pneumonia, dyspnea, insomnia, bronchitis, cough, and back pain. The most common hematology laboratory abnormalities (≥80%) are decreased hemoglobin, decreased lymphocytes, and decreased platelets. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact sanofi-aventis U.S. LLC at 1-800-633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2022
Related/similar drugs
Darzalex, Blenrep, Tecvayli, Revlimid, Velcade, Pomalyst, KyprolisFull Prescribing Information
1. Indications and Usage for Sarclisa
SARCLISA is indicated:
- in combination with pomalidomide and dexamethasone, for the treatment of adult patients with multiple myeloma who have received at least 2 prior therapies including lenalidomide and a proteasome inhibitor.
- in combination with carfilzomib and dexamethasone, for the treatment of adult patients with relapsed or refractory multiple myeloma who have received 1 to 3 prior lines of therapy.
2. Sarclisa Dosage and Administration
2.1 Recommended Dosage
- Administer pre-infusion medications [see Dosage and Administration (2.2)].
- SARCLISA should be administered by a healthcare professional, with immediate access to emergency equipment and appropriate medical support to manage infusion-related reactions if they occur [see Warnings and Precautions (5.1)].
The recommended dose of SARCLISA is 10 mg/kg actual body weight administered as an intravenous infusion in combination with pomalidomide and dexamethasone or in combination with carfilzomib and dexamethasone, according to the schedule in Table 1 [see Clinical Studies (14)].
| Cycle | Dosing schedule |
|---|---|
| Cycle 1 | Days 1, 8, 15, and 22 (weekly) |
| Cycle 2 and beyond | Days 1, 15 (every 2 weeks) |
Each treatment cycle consists of a 28-day period. Treatment is repeated until disease progression or unacceptable toxicity.
SARCLISA is used in combination with pomalidomide and dexamethasone or in combination with carfilzomib and dexamethasone. For dosing instructions of combination agents administered with SARCLISA, see Clinical Studies (14) and manufacturer's prescribing information.
2.2 Recommended Premedications and Antiviral Prophylaxis
Administer the following premedications prior to SARCLISA infusion to reduce the risk and severity of infusion-related reactions [see Warnings and Precautions (5.1)]:
- When administered in combination with SARCLISA and pomalidomide: Dexamethasone 40 mg orally or intravenously (or 20 mg orally or intravenously for patients ≥75 years of age).
When administered in combination with SARCLISA and carfilzomib: Dexamethasone 20 mg (intravenously on the days of SARCLISA and/or carfilzomib infusions, orally on day 22 in cycle 2 and beyond, and orally on day 23 in all cycles). - Acetaminophen 650 mg to 1,000 mg orally (or equivalent).
- H2 antagonists
- Diphenhydramine 25 mg to 50 mg orally or intravenously (or equivalent). The intravenous route is preferred for at least the first 4 infusions.
The above recommended dose of dexamethasone (orally or intravenously) corresponds to the dose to be administered before infusion as part of the premedication and part of the backbone treatment. Administer dexamethasone before SARCLISA and pomalidomide and before SARCLISA and carfilzomib administration.
Administer the recommended premedication agents 15 to 60 minutes prior to starting a SARCLISA infusion.
2.3 Dose Modifications
No dose reduction of SARCLISA is recommended. Dose delay may be required to allow recovery of blood counts in the event of hematological toxicity [see Warnings and Precautions (5.2, 5.4)]. For information concerning drugs given in combination with SARCLISA, see manufacturer's prescribing information.
2.4 Preparation
Prepare the solution for infusion using aseptic technique as follows:
Calculate the dose (mg) of required SARCLISA based on actual patient weight (measured prior to each cycle to have the administered dose adjusted accordingly) [see Dosage and Administration (2.1)]. More than one SARCLISA vial may be necessary to obtain the required dose for the patient.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Remove the volume of diluent from the 250 mL Sodium Chloride Injection, USP, or 5% Dextrose Injection, USP diluent bag that is equal to the required volume of SARCLISA injection.
- Withdraw the necessary volume of SARCLISA injection from the vial and dilute by adding to the infusion bag of 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP.
- The infusion bag must be made of polyolefins (PO), polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC) with di-(2-ethylhexyl) phthalate (DEHP) or ethyl vinyl acetate (EVA).
- Gently homogenize the diluted solution by inverting the bag. Do not shake.
2.5 Administration
- Administer the infusion solution by intravenous infusion using an intravenous tubing infusion set (in PE, PVC with or without DEHP, polybutadiene [PBD], or polyurethane [PU]) with a 0.22 micron in-line filter (polyethersulfone [PES], polysulfone, or nylon).
- The infusion solution should be administered for a period of time that will depend on the infusion rate (see Table 2). Use prepared SARCLISA infusion solution within 48 hours when stored refrigerated at 2°C–8°C, followed by 8 hours (including the infusion time) at room temperature.
- Do not administer SARCLISA infusion solution concomitantly in the same intravenous line with other agents.
- On the days where both SARCLISA and carfilzomib are administered, administer dexamethasone first, followed by SARCLISA infusion, then followed by carfilzomib infusion.
Infusion Rates
Following dilution, administer the SARCLISA infusion solution intravenously at the infusion rates presented in Table 2. Incremental escalation of the infusion rate should be considered only in the absence of infusion-related reactions [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
| Dilution Volume | Initial Rate | Absence of Infusion-Related Reaction | Rate Increment | Maximum Rate | |
|---|---|---|---|---|---|
| First infusion | 250 mL | 25 mL/hour | For 60 minutes | 25 mL/hour every 30 minutes | 150 mL/hour |
| Second infusion | 250 mL | 50 mL/hour | For 30 minutes | 50 mL/hour for 30 minutes then increase by 100 mL/hour | 200 mL/hour |
| Subsequent infusions | 250 mL | 200 mL/hour | – | – | 200 mL/hour |
3. Dosage Forms and Strengths
SARCLISA is a clear to slightly opalescent, colorless to slightly yellow solution, essentially free of visible particulates available as:
- Injection: 100 mg/5 mL (20 mg/mL) in a single-dose vial
- Injection: 500 mg/25 mL (20 mg/mL) in a single-dose vial
4. Contraindications
SARCLISA is contraindicated in patients with severe hypersensitivity to isatuximab-irfc or to any of its excipients [see Warnings and Precautions (5.1)].
5. Warnings and Precautions
5.1 Infusion-Related Reactions
Serious infusion-related reactions including life-threatening anaphylactic reactions have occurred with SARCLISA treatment. Severe signs and symptoms included cardiac arrest, hypertension, hypotension, bronchospasm, dyspnea, angioedema, and swelling.
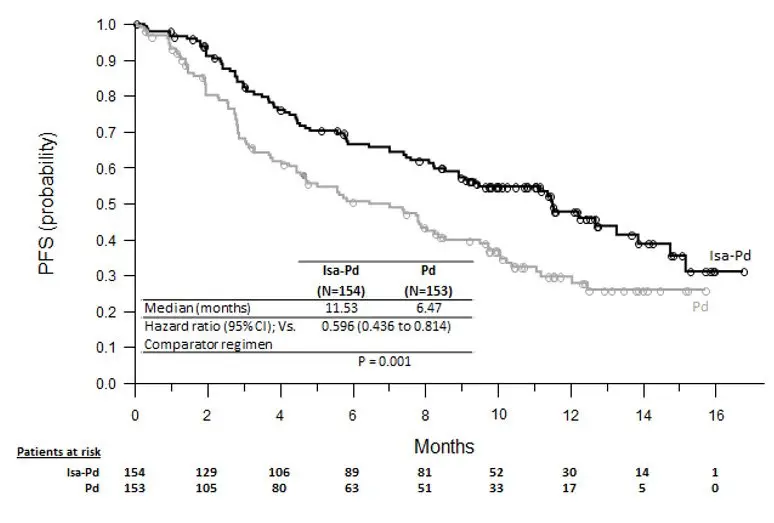
Based on ICARIA-MM, infusion-related reactions occurred in 38% of patients treated with SARCLISA, pomalidomide, and dexamethasone (Isa-Pd) [see Adverse Reactions (6.1)]. All infusion-related reactions started during the first SARCLISA infusion and resolved on the same day in 98% of the cases.
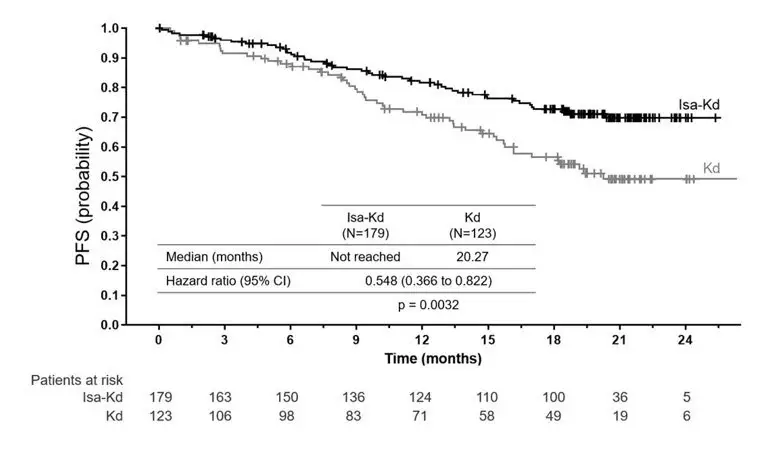
In IKEMA, infusion-related reactions occurred in 46% of patients treated with SARCLISA, carfilzomib, and dexamethasone (Isa-Kd). In the Isa-Kd arm, the infusion-related reactions occurred on the infusion day in 99% of episodes. In patients treated with Isa-Kd, 95% of those experiencing an infusion-related reaction experienced it during the first cycle of treatment. All infusion-related reactions resolved: within the same day in 74% of episodes, and the day after in 24% of episodes [see Adverse Reactions (6.1)].
The most common symptoms (≥5%) of an infusion-related reaction in ICARIA-MM and IKEMA (N=329) included dyspnea, cough, nasal congestion, and nausea. Anaphylactic reactions occurred in less than 1% of patients.
To decrease the risk and severity of infusion-related reactions, premedicate patients prior to SARCLISA infusion with acetaminophen, H2 antagonists, diphenhydramine, or equivalent, and dexamethasone [see Dosage and Administration (2.2)].
Monitor vital signs frequently during the entire SARCLISA infusion. For patients with grade ≥2 reactions, interrupt SARCLISA infusion and provide appropriate medical management. For patients with grade 2 or grade 3 reactions, if symptoms improve to grade ≤1, restart SARCLISA infusion at half of the initial infusion rate, with supportive care as needed, and closely monitor patients. If symptoms do not recur after 30 minutes, the infusion rate may be increased to the initial rate, and then increased incrementally, as shown in Table 2 [see Dosage and Administration (2.5)]. In case symptoms do not improve to grade ≤1 after interruption of SARCLISA infusion, persist or worsen despite appropriate medications, or require hospitalization, permanently discontinue SARCLISA and institute appropriate management. Permanently discontinue SARCLISA if an anaphylactic reaction or life-threatening (grade 4) infusion-related reaction occurs and institute appropriate management.
5.2 Neutropenia
SARCLISA may cause neutropenia.
In patients treated with Isa-Pd, neutropenia occurred in 96% of patients and grade 3–4 neutropenia occurred in 85% of patients. Neutropenic complications occurred in 30% of patients, including febrile neutropenia (12%) and neutropenic infections (25%), defined as infection with concurrent grade ≥3 neutropenia. The most frequent neutropenic infections included infections of the upper respiratory tract (10%), lower respiratory tract (9%), and urinary tract (3%) [see Adverse Reactions (6.1)].
In patients treated with Isa-Kd, neutropenia occurred in 55% of patients, with grade 3–4 neutropenia in 19% of patients (grade 3 in 18% and grade 4 in 1.7%). Neutropenic complications occurred in 2.8% of patients, including febrile neutropenia (1.1%) and neutropenic infections (1.7%) [see Adverse Reactions (6.1)].
Monitor complete blood cell counts periodically during treatment. Consider the use of antibiotics and antiviral prophylaxis during treatment [see Dosage and Administration (2.2)]. Monitor patients with neutropenia for signs of infection. In case of grade 4 neutropenia delay SARCLISA dose until neutrophil count recovery to at least 1.0 × 109/L, and provide supportive care with growth factors, according to institutional guidelines. No dose reductions of SARCLISA are recommended.
5.3 Second Primary Malignancies
The incidence of second primary malignancies is increased in patients treated with SARCLISA-containing regimens. The overall incidence of second primary malignancies in all the SARCLISA-exposed patients was 3.6%.
In ICARIA-MM, second primary malignancies occurred in 3.9% of patients in the Isa-Pd arm and in 0.7% of patients in the Pd arm.
In IKEMA, second primary malignancies occurred in 7% of patients in the Isa-Kd arm and in 4.9% of patients in the Kd arm.
The most common (≥1%) second primary malignancies in ICARIA-MM and IKEMA (N=329) included skin cancers (4% with SARCLISA-containing regimens and 1.5% with comparative regimens) and solid tumors other than skin cancer (1.8% with SARCLISA-containing regimens and 1.5% with comparative regimens). All patients with skin cancer continued treatment after resection of the skin cancer.
Monitor patients for the development of second primary malignancies.
5.5 Embryo-Fetal Toxicity
Based on the mechanism of action, SARCLISA can cause fetal harm when administered to a pregnant woman. SARCLISA may cause fetal immune cell depletion and decreased bone density. Advise pregnant women of the potential risk to a fetus. Advise females with reproductive potential to use an effective method of contraception during treatment with SARCLISA and for 5 months after the last dose [see Use in Specific Populations (8.1, 8.3)]. The combination of SARCLISA with pomalidomide is contraindicated in pregnant women because pomalidomide may cause birth defects and death of the unborn child. Refer to the pomalidomide prescribing information on use during pregnancy.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions from SARCLISA are also described in other sections of the labeling:
- Infusion-Related Reactions [see Warnings and Precautions (5.1)]
- Neutropenia [see Warnings and Precautions (5.2)]
- Second Primary Malignancies [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Multiple Myeloma
Combination treatment with pomalidomide and dexamethasone (Isa-Pd)
The safety of SARCLISA was evaluated in ICARIA-MM, a randomized, open-label clinical trial in patients with previously treated multiple myeloma. Patients received SARCLISA 10 mg/kg intravenously, weekly in the first cycle and every two weeks thereafter, in combination with pomalidomide and dexamethasone (Isa-Pd) (n=152) or pomalidomide and dexamethasone (Pd) (n=149) [see Clinical Studies (14)]. Among patients receiving Isa-Pd, 66% were exposed to SARCLISA for 6 months or longer and 24% were exposed for greater than 12 months or longer.
Serious adverse reactions occurred in 62% of patients receiving Isa-Pd. Serious adverse reactions in >5% of patients who received Isa-Pd included pneumonia (26%), upper respiratory tract infections (7%), and febrile neutropenia (7%). Fatal adverse reactions occurred in 11% of patients (those that occurred in more than 1% of patients were pneumonia and other infections [3%]).
Permanent treatment discontinuation due to an adverse reaction (grades 1–4) occurred in 7% of patients who received Isa-Pd. The most frequent adverse reactions requiring permanent discontinuation in patients who received Isa-Pd were infections (2.6%). SARCLISA alone was discontinued in 3% of patients due to infusion-related reactions.
Dosage interruptions due to an adverse reaction occurred in 31% of patients who received SARCLISA. The most frequent adverse reaction requiring dosage interruption was infusion-related reaction (28%).
The most common adverse reactions (≥20%) were upper respiratory tract infection, infusion-related reactions, pneumonia, and diarrhea.
Table 3 summarizes the adverse reactions in ICARIA-MM.
| Adverse Reactions | SARCLISA + Pomalidomide + Dexamethasone (Isa-Pd) | Pomalidomide + Dexamethasone (Pd) | ||||
|---|---|---|---|---|---|---|
| (N=152) | (N=149) | |||||
| All Grades (%) | Grade 3 (%) | Grade 4 (%) | All Grades (%) | Grade 3 (%) | Grade 4 (%) |
|
| CTCAE version 4.03 | ||||||
|
||||||
| General disorders and administration site conditions | ||||||
| Infusion-related reaction* | 38 | 1.3 | 1.3 | 0 | 0 | 0 |
| Infections | ||||||
| Upper respiratory tract infection† | 57 | 9 | 0 | 42 | 3.4 | 0 |
| Pneumonia‡ | 31 | 22 | 3.3 | 23 | 16 | 2.7 |
| Blood and lymphatic system disorders | ||||||
| Febrile neutropenia | 12 | 11 | 1.3 | 2 | 1.3 | 0.7 |
| Respiratory, thoracic and mediastinal disorders | ||||||
| Dyspnea§ | 17 | 5 | 0 | 12 | 1.3 | 0 |
| Gastrointestinal disorders | ||||||
| Diarrhea | 26 | 2 | 0 | 19 | 0.7 | 0 |
| Nausea | 15 | 0 | 0 | 9 | 0 | 0 |
| Vomiting | 12 | 1.3 | 0 | 3.4 | 0 | 0 |
Table 4 summarizes the hematology laboratory abnormalities in ICARIA-MM.
| Laboratory Parameter | SARCLISA + Pomalidomide + Dexamethasone (Isa-Pd) | Pomalidomide + Dexamethasone (Pd) | ||||
|---|---|---|---|---|---|---|
| (N=152) | (N=149) | |||||
| All Grades (%) | Grade 3 (%) | Grade 4 (%) | All Grades (%) | Grade 3 (%) | Grade 4 (%) |
|
| The denominator used to calculate the percentages was based on the safety population. | ||||||
| Hemoglobin decreased | 99 | 32 | 0 | 97 | 28 | 0 |
| Neutrophils decreased | 96 | 24 | 61 | 92 | 38 | 31 |
| Lymphocytes decreased | 92 | 42 | 13 | 92 | 35 | 8 |
| Platelets decreased | 84 | 14 | 16 | 79 | 9 | 15 |
Combination treatment with carfilzomib and dexamethasone (Isa-Kd)
The safety of SARCLISA was evaluated in IKEMA, a randomized, open-label clinical trial in patients with previously treated multiple myeloma. Patients received SARCLISA 10 mg/kg intravenously weekly in the first cycle, and every two weeks thereafter, in combination with carfilzomib and dexamethasone (Isa-Kd) (n=177) or carfilzomib and dexamethasone (Kd) (n=122) [see Clinical Studies (14)]. Among patients receiving Isa-Kd, 68% were exposed to SARCLISA for 12 months or longer and 51% were exposed for greater than 18 months.
Serious adverse reactions occurred in 59% of patients receiving Isa-Kd. The most frequent serious adverse reactions in >5% of patients who received Isa-Kd were pneumonia (25%) and upper respiratory tract infections (9%). Adverse reactions with a fatal outcome during treatment were reported in 3.4% of patients in the Isa-Kd group (those occurring in more than 1% of patients were pneumonia occurring in 1.7% and cardiac failure in 1.1% of patients).
Permanent treatment discontinuation due to an adverse reaction (grades 1–4) occurred in 8% of patients who received Isa-Kd. The most frequent adverse reactions requiring permanent discontinuation in patients who received Isa-Kd were infections (2.8%). SARCLISA alone was discontinued in 0.6% of patients due to infusion-related reactions.
Dosage interruptions due to an adverse reaction occurred in 33% of patients who received SARCLISA. The most frequent adverse reaction requiring dosage interruption was infusion-related reaction (30%).
The most common adverse reactions (≥20%) were upper respiratory tract infection, infusion-related reactions, fatigue, hypertension, diarrhea, pneumonia, dyspnea, insomnia, bronchitis, cough, and back pain.
Table 5 summarizes the adverse reactions in IKEMA.
| Adverse Reactions | SARCLISA + Carfilzomib + Dexamethasone (Isa-Kd) | Carfilzomib + Dexamethasone (Kd) | ||||
|---|---|---|---|---|---|---|
| (N=177) | (N=122) | |||||
| All Grades (%) | Grade 3 (%) | Grade 4 (%) | All Grades (%) | Grade 3 (%) | Grade 4 (%) |
|
|
||||||
| General disorders and administration site conditions | ||||||
| Infusion-related reaction* | 46 | 0.6 | 0 | 3.3 | 0 | 0 |
| Fatigue† | 42 | 5 | 0 | 32 | 3.3 | 0 |
| Infections | ||||||
| Upper respiratory tract infection‡ | 67 | 9 | 0 | 57 | 7 | 0 |
| Pneumonia§ | 36 | 19 | 3.4 | 30 | 15 | 2.5 |
| Bronchitis¶ | 24 | 2.3 | 0 | 13 | 0.8 | 0 |
| Vascular disorders | ||||||
| Hypertension# | 37 | 20 | 0.6 | 32 | 18 | 1.6 |
| Respiratory, thoracic and mediastinal disorders | ||||||
| DyspneaÞ | 29 | 5 | 0 | 24 | 0.8 | 0 |
| Coughß | 23 | 0 | 0 | 15 | 0 | 0 |
| Gastrointestinal disorders | ||||||
| Diarrhea | 36 | 2.8 | 0 | 29 | 2.5 | 0 |
| Vomiting | 15 | 1.1 | 0 | 9 | 0.8 | 0 |
Table 6 summarizes the hematology laboratory abnormalities in IKEMA.
| Laboratory Parameter | SARCLISA + Carfilzomib + Dexamethasone (Isa-Kd) | Carfilzomib + Dexamethasone (Kd) | ||||
|---|---|---|---|---|---|---|
| (N=177) | (N=122) | |||||
| All Grades (%) | Grade 3 (%) | Grade 4 (%) | All Grades (%) | Grade 3 (%) | Grade 4 (%) |
|
| The denominator used to calculate the percentage was based on the safety population. | ||||||
| Hemoglobin decreased | 99 | 22 | 0 | 99 | 20 | 0 |
| Lymphocytes decreased | 94 | 52 | 17 | 95 | 43 | 14 |
| Platelets decreased | 94 | 19 | 11 | 88 | 16 | 8 |
| Neutrophils decreased | 55 | 18 | 1.7 | 43 | 7 | 0.8 |
6.2 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other isatuximab-irfc products may be misleading.
In ICARIA-MM and IKEMA, no patients tested positive for antidrug antibodies (ADA). Therefore, the neutralizing ADA status was not determined. Overall, across 9 clinical studies in multiple myeloma (MM) with SARCLISA single-agent and combination therapies including ICARIA-MM and IKEMA (N=1018), the incidence of treatment emergent ADAs was 1.9%. No clinically significant differences in the pharmacokinetics, safety, or efficacy of isatuximab-irfc were observed in patients with ADAs.
8. Use In Specific Populations
8.3 Females and Males of Reproductive Potential
SARCLISA can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
8.5 Geriatric Use
Of the total number of subjects in clinical studies of SARCLISA, 56% (586 patients) were 65 and over, while 16% (163 patients) were 75 and over. No overall differences in safety or effectiveness were observed between subjects 65 and over and younger subjects, and other reported clinical experience has not identified differences in responses between the adults 65 years and over and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
11. Sarclisa Description
Isatuximab-irfc, a CD38-directed cytolytic antibody, is a chimeric immunoglobulin G1 (IgG1) monoclonal antibody (mAb). Isatuximab-irfc is produced from a mammalian cell line (Chinese hamster ovary, CHO) using a fed-batch production process. Isatuximab-irfc is composed of two identical immunoglobulin kappa light chains and two identical immunoglobulin gamma heavy chains and has an overall molecular weight of approximately 148 kDa.
SARCLISA (isatuximab-irfc) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution, essentially free of visible particles in a single-dose vial for intravenous use. Each vial contains either 100 mg/5 mL or 500 mg/25 mL of isatuximab-irfc at a concentration of 20 mg/mL with a pH of 6.0. Each mL of solution contains 20 mg isatuximab-irfc, histidine (1.46 mg), histidine hydrochloride monohydrate (2.22 mg), polysorbate 80 (0.2 mg), sucrose (100 mg), and water for injection.
12. Sarclisa - Clinical Pharmacology
12.1 Mechanism of Action
Isatuximab-irfc is an IgG1-derived monoclonal antibody that binds to CD38 expressed on the surface of hematopoietic and tumor cells, including multiple myeloma cells. Isatuximab-irfc induces apoptosis of tumor cells and activation of immune effector mechanisms including antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement dependent cytotoxicity (CDC). Isatuximab-irfc inhibits the ADP-ribosyl cyclase activity of CD38. Isatuximab-irfc can activate natural killer (NK) cells in the absence of CD38-positive target tumor cells and suppresses CD38-positive T-regulatory cells. The combination of isatuximab-irfc and pomalidomide enhanced ADCC activity and direct tumor cell killing compared to that of isatuximab-irfc alone in vitro, and enhanced antitumor activity compared to the activity of isatuximab-irfc or pomalidomide alone in a human multiple myeloma xenograft model.
12.2 Pharmacodynamics
In multiple myeloma patients treated with SARCLISA combined with pomalidomide and dexamethasone, a decrease in absolute counts of total NK cells (including inflammatory CD16+ low CD56+ bright and cytotoxic CD16+ bright CD56+ dim NK cells) and CD19+ B cells was observed in peripheral blood.
12.3 Pharmacokinetics
Following administration of isatuximab-irfc in combination with pomalidomide and dexamethasone at the recommended dose and schedule, the steady-state mean (CV%) predicted maximum plasma concentration (Cmax) and area under the plasma concentration-time curve (AUC) of isatuximab-irfc were 351 µg/mL (36.0%) and 72,600 µg∙h/mL (51.7%), respectively.
Following administration of isatuximab-irfc in combination with carfilzomib and dexamethasone at the recommended dose and schedule, the steady state mean (CV%) predicted Cmax and AUC of isatuximab-irfc were 655 µg/mL (30.8%) and 159,000 µg∙h/mL (37.1%), respectively.
The median time to reach steady state of isatuximab-irfc was 18 weeks with a 3.1-fold accumulation.
Isatuximab-irfc AUC increases in a greater than dose proportional manner over a dosage range from 1 mg/kg to 20 mg/kg (0.1 to 2 times the approved recommended dosage) every 2 weeks. Isatuximab-irfc AUC increases proportionally over a dosage range from 5 mg/kg to 20 mg/kg (0.5 to 2 times the approved recommended dosage) every week for 4 weeks followed by every 2 weeks.
| SARCLISA
isatuximab injection, solution, concentrate |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| SARCLISA
isatuximab injection, solution, concentrate |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - sanof-aventis U.S. LLC (824676584) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi-Aventis Deutschland GmbH | 313218430 | MANUFACTURE(0024-0654, 0024-0656) , ANALYSIS(0024-0654, 0024-0656) , LABEL(0024-0654, 0024-0656) , PACK(0024-0654, 0024-0656) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genzyme Corporation | 050424395 | LABEL(0024-0654, 0024-0656) , PACK(0024-0654, 0024-0656) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi Chimie | 291592785 | API MANUFACTURE(0024-0654, 0024-0656) , ANALYSIS(0024-0654, 0024-0656) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Quality Assistance, S.A. | 283676641 | ANALYSIS(0024-0654, 0024-0656) | |