Drug Detail:Sivextro (Tedizolid (oral/injection) [ te-diz-oh-lid ])
Drug Class: Oxazolidinone antibiotics
Highlights of Prescribing Information
SIVEXTRO® (tedizolid phosphate) for injection, for intravenous use
SIVEXTRO® (tedizolid phosphate) tablet, for oral use
Initial U.S. Approval: 2014
Indications and Usage for Sivextro
SIVEXTRO is an oxazolidinone antibacterial indicated in adult and pediatric patients 12 years of age and older for the treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by designated susceptible bacteria. (1.1)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of SIVEXTRO and other antibacterial drugs, SIVEXTRO should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
Sivextro Dosage and Administration
200 mg administered once daily orally or as an intravenous (IV) infusion over 1 hour for six (6) days in adult and pediatric patients 12 years of age and older. (2.1)
Dosage Forms and Strengths
- For injection: 200 mg, sterile, lyophilized powder in single-dose vial for reconstitution for intravenous infusion. (3)
- Tablet: 200 mg (3)
Contraindications
None (4)
Warnings and Precautions
- Patients with neutropenia: The safety and efficacy of SIVEXTRO in patients with neutropenia (neutrophil counts <1000 cells/mm3) have not been adequately evaluated. In an animal model of infection, the antibacterial activity of SIVEXTRO was reduced in the absence of granulocytes. Consider alternative therapies in neutropenic patients. (5.1)
- Clostridioides difficile-associated diarrhea: Evaluate if diarrhea occurs. (5.2)
Adverse Reactions/Side Effects
The most common adverse reactions (≥2%) in adults are nausea, headache, diarrhea, infusion- or injection-related adverse reactions, vomiting, and dizziness. The most common adverse reactions (≥2%) in pediatric patients are phlebitis, and increased hepatic transaminases. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
Drug Interactions
SIVEXTRO (when administered orally) can increase the plasma concentrations of orally administered Breast Cancer Resistance Protein (BCRP) substrates. Monitor for adverse reactions related to the concomitant BCRP substrates if coadministration cannot be avoided. (7, 12.3)
Use In Specific Populations
Pregnancy: Based on animal data, SIVEXTRO may cause fetal harm. Advise pregnant women of the potential risks to a fetus. (8.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2022
Related/similar drugs
amoxicillin, doxycycline, ciprofloxacin, cephalexin, metronidazole, azithromycin, AugmentinFull Prescribing Information
1. Indications and Usage for Sivextro
1.1 Acute Bacterial Skin and Skin Structure Infections
SIVEXTRO® is an oxazolidinone-class antibacterial indicated in adult and pediatric patients 12 years of age and older for the treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by susceptible isolates of the following Gram-positive microorganisms: Staphylococcus aureus (including methicillin-resistant [MRSA] and methicillin-susceptible [MSSA] isolates), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus anginosus Group (including Streptococcus anginosus, Streptococcus intermedius, and Streptococcus constellatus), and Enterococcus faecalis.
1.2 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of SIVEXTRO and other antibacterial drugs, SIVEXTRO should be used only to treat ABSSSI that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
2. Sivextro Dosage and Administration
2.1 Recommended Dosage
The recommended dosage of SIVEXTRO is 200 mg administered once daily for six (6) days either orally (with or without food) or as an intravenous (IV) infusion in patients 12 years of age or older.
The recommended dosage and administration of SIVEXTRO are described in Table 1.
| Infection | Route | Dosage | Frequency | Infusion Time | Duration of Treatment |
|---|---|---|---|---|---|
| Acute Bacterial Skin and Skin Structure Infections (ABSSSI) | Intravenous | 200 mg | Once daily | 1 hour | 6 days |
| Oral | 200 mg | Once daily | Not Applicable |
No dose adjustment is necessary when changing from intravenous to oral SIVEXTRO.
If patients miss a dose, they should take it as soon as possible anytime up to 8 hours prior to their next scheduled dose. If less than 8 hours remain before the next dose, wait until their next scheduled dose.
2.2 Preparation and Administration of Intravenous Solution
SIVEXTRO is supplied as a sterile, lyophilized powder for injection in single-dose vials of 200 mg. Each 200 mg vial must be reconstituted with Sterile Water for Injection and subsequently diluted only with 0.9% Sodium Chloride Injection, USP.
SIVEXTRO vials contain no antimicrobial preservatives and are intended for single dose only. Discard any unused portion.
2.3 Compatible Intravenous Solutions
SIVEXTRO is compatible with 0.9% Sodium Chloride Injection, USP.
2.4 Incompatibilities
SIVEXTRO for injection is incompatible with any solution containing divalent cations (e.g., Ca2+, Mg2+), including Lactated Ringer's Injection and Hartmann's Solution.
Limited data are available on the compatibility of SIVEXTRO for injection with other intravenous substances, additives or other medications and they should not be added to SIVEXTRO single-dose vials or infused simultaneously. If the same intravenous line is used for sequential infusion of several different drugs, the line should be flushed before and after infusion of SIVEXTRO with 0.9% Sodium Chloride Injection, USP.
3. Dosage Forms and Strengths
SIVEXTRO 200 mg tablet is a yellow film-coated oval tablet; each tablet is debossed with "TZD" on one side and "200" on the other side.
SIVEXTRO for injection is a sterile, white to off-white lyophilized powder for injection in single-dose vials of 200 mg. Each 200 mg vial must be reconstituted with Sterile Water for Injection and subsequently diluted only with 0.9% Sodium Chloride Injection, USP.
5. Warnings and Precautions
5.1 Patients with Neutropenia
The safety and efficacy of SIVEXTRO in patients with neutropenia (neutrophil counts <1000 cells/mm3) have not been adequately evaluated. In an animal model of infection, the antibacterial activity of SIVEXTRO was reduced in the absence of granulocytes [see Clinical Pharmacology (12.2)]. Alternative therapies should be considered when treating patients with neutropenia and ABSSSI.
5.2 Clostridioides difficile-Associated Diarrhea
Clostridioides difficile-associated diarrhea (CDAD) has been reported for nearly all systemic antibacterial agents including SIVEXTRO, with severity ranging from mild diarrhea to fatal colitis. Treatment with antibacterial agents can alter the normal flora of the colon and may permit overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antibacterial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary because CDAD has been reported to occur more than two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, antibacterial use not directed against C. difficile should be discontinued, if possible. Appropriate measures such as fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be compared directly to rates from clinical trials of another drug and may not reflect rates observed in practice.
Adult Patients
Adverse reactions were evaluated for 1425 adult patients treated with SIVEXTRO in two Phase 2 and four Phase 3 clinical trials (three Phase 3 trials for 6 days of therapy and one Phase 3 trial for 7-21 days of therapy). The median age of adult patients treated with SIVEXTRO in the Phase 2 and Phase 3 trials was 44 years, ranging between 17 and 94 years old. The majority of adult patients treated with SIVEXTRO were male (66%) and White (67%).
Most Common Adverse Reactions in Adults
The most common adverse reactions in adult patients treated with SIVEXTRO were nausea (7.1%), headache (4.5%), diarrhea (3.6%), vomiting (2.7%), and dizziness (1.6%). The median time of onset of adverse reactions was 5 days for both SIVEXTRO and linezolid with 12% occurring on the second day of treatment in both treatment groups.
Table 2 lists selected adverse reactions occurring in at least 2% of adult patients treated with SIVEXTRO in clinical trials.
| Adverse Reactions | Pooled Phase 3 ABSSSI Clinical Trials | |
|---|---|---|
| SIVEXTRO (200 mg oral/intravenous once daily for 6 days) (N=1037) | Linezolid (600 mg oral/intravenous twice daily for 10 days) (N=1000) |
|
|
||
| Gastrointestinal Disorders | ||
| Nausea | 7% | 10% |
| Diarrhea | 4% | 5% |
| Vomiting | 3% | 5% |
| Nervous System Disorder | ||
| Headache | 5% | 5% |
| Dizziness | 2% | 2% |
| Infusion- or Injection-Related Adverse Reactions* | ||
| 4% | 2% | |
The following selected adverse reactions were reported in SIVEXTRO-treated adult patients at a rate of less than 2% in these clinical trials:
Blood and Lymphatic System Disorders: anemia
Cardiovascular: palpitations, tachycardia
Eye Disorders: asthenopia, vision blurred, visual impairment, vitreous floaters
Immune System Disorders: drug hypersensitivity
Infections and Infestations: Clostridioides difficile colitis, oral candidiasis, vulvovaginal mycotic infection
Investigations: hepatic transaminases increased (ALT increased, AST increased), gamma-glutamyltransferase (GGT) increased, white blood cell count decreased
Nervous System Disorders: hypoesthesia, paresthesia, VIIth nerve paralysis
Psychiatric Disorders: insomnia
Skin and Subcutaneous Tissue Disorders: pruritus, urticaria, dermatitis
Vascular Disorders: flushing, hypertension
Laboratory Parameters
Hematology laboratory abnormalities that were determined to be potentially clinically significant in the pooled Phase 3 ABSSSI clinical trials are provided in Table 3.
| Laboratory Assay | Potentially Clinically Significant Values*,† | |
|---|---|---|
| SIVEXTRO (200 mg oral/intravenous once daily for 6 days) (N)‡ | Linezolid (600 mg oral/intravenous twice daily for 10 days) (N)‡ |
|
| M = male; F = female | ||
|
||
| Hemoglobin (<10.1 g/dL [M]) (<9 g/dL [F]) | (994) 3.4% | (957) 3.4% |
| Platelet count (<112 × 103/mm3) | (989) 2.1% | (950) 3.8% |
| Absolute neutrophil count (<0.8 × 103/mm3) | (980) 0.4% | (941) 0.6% |
Peripheral and Optic Neuropathy
Peripheral and optic neuropathy have been described in patients treated with another member of the oxazolidinone class for longer than 28 days. In Phase 3 trials in adults, reported adverse reactions for peripheral neuropathy and optic nerve disorders were similar between both treatment arms (peripheral neuropathy 1.2% vs. 0.7% for tedizolid phosphate and linezolid, respectively; optic nerve disorders 0.3% vs. 0.1%, respectively).
Pediatric Patients
Adverse reactions were evaluated in 91 pediatric patients with ABSSSI ranging from 12 to <18 years of age treated with IV and/or oral SIVEXTRO 200 mg for 6 days and 29 patients treated with comparator agents for 10 days. The majority of pediatric patients treated with SIVEXTRO were male (64%) and white (88%).
Serious adverse reactions occurred in 1/91 (1%) of pediatric patients treated with SIVEXTRO and in none of the 29 patients treated with the comparator. Adverse reactions leading to discontinuation occurred in 1 (1%) pediatric patient in the SIVEXTRO arm and in none in the comparator arm.
The most common adverse reactions occurring in pediatric patients receiving SIVEXTRO in the ABSSSI clinical trial were phlebitis (3%), increased hepatic transaminases (alanine aminotransferase, aspartate aminotransferase) (3%), anemia, and vomiting (1%).
Safety has not been evaluated in pediatric patients under 12 years of age.
Laboratory Parameters
| Laboratory Assay | Potentially Clinically Significant Values*,† | |
|---|---|---|
| SIVEXTRO (200 mg oral/intravenous once daily for 6 days) (N)‡ | Comparators§
(for 10 days) (N)‡ |
|
| M = male; F = female | ||
|
||
| Hemoglobin (<10.1 g/dL [M]) (<9 g/dL [F]) | (85) 2.4% | (26) 0.0% |
| Platelet count (<112 × 103/mm3) | (82) 1.2% | (26) 0.0% |
| Absolute neutrophil count (<0.8 × 103/mm3) | (85) 0.0% | (26) 0.0% |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of SIVEXTRO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: thrombocytopenia
7. Drug Interactions
Orally administered SIVEXTRO inhibits Breast Cancer Resistance Protein (BCRP) in the intestine, which can increase the plasma concentrations of orally administered BCRP substrates, and the potential for adverse reactions. If possible, an interruption in the treatment of the co-administered BCRP substrate medicinal product should be considered during treatment with SIVEXTRO, especially for BCRP substrates with a narrow therapeutic index (e.g., methotrexate or topotecan). If coadministration cannot be avoided, monitor for adverse reactions related to the concomitantly administered BCRP substrates, including rosuvastatin. [See Clinical Pharmacology (12.3).]
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of SIVEXTRO for the treatment of ABSSSI have been established in pediatric patients aged 12 years and older. Use of SIVEXTRO for the treatment of ABSSSI is supported by evidence from adequate and well-controlled studies in adults with additional pharmacokinetic and safety data in pediatric patients aged 12 years and older [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.1)].
Safety and effectiveness of SIVEXTRO in pediatric patients below the age of 12 years have not been established.
10. Overdosage
In the event of overdosage, SIVEXTRO should be discontinued and general supportive treatment given. Hemodialysis does not result in meaningful removal of tedizolid from systemic circulation.
11. Sivextro Description
SIVEXTRO (tedizolid phosphate), a phosphate prodrug, is converted to tedizolid in the presence of phosphatases.
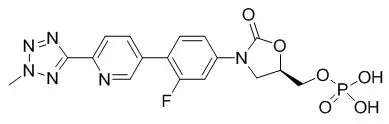
Tedizolid phosphate has the chemical name [(5R)-(3-{3-Fluoro-4-[6-(2-methyl-2H-tetrazol- 5-yl) pyridin-3-yl]phenyl}-2-oxooxazolidin- 5-yl]methyl hydrogen phosphate.
Its empirical formula is C17H16FN6O6P and its molecular weight is 450.32. Its structural formula is:
Tedizolid phosphate is a white to yellow solid and is administered orally or by intravenous infusion.
The pharmacologically active moiety, tedizolid, is an antibacterial agent of the oxazolidinone class.
SIVEXTRO tablets contain 200 mg of tedizolid phosphate, and the following inactive ingredients: crospovidone, magnesium stearate, mannitol, microcrystalline cellulose, and povidone. In addition, the film coating contains the following inactive ingredients: polyethylene glycol/macrogol, polyvinyl alcohol, talc, titanium dioxide, and yellow iron oxide.
SIVEXTRO for injection is a sterile, white to off-white sterile lyophilized powder supplied in a clear glass single-dose vial. Each vial contains 200 mg of tedizolid phosphate and the inactive ingredient, mannitol (105 mg). Sodium hydroxide and hydrochloric acid are used as needed for pH adjustment. When reconstituted as directed with 4 mL of Sterile Water for Injection, each mL contains 50 mg of tedizolid phosphate. The pH of the reconstituted solution is 7.4 to 8.1.
12. Sivextro - Clinical Pharmacology
12.2 Pharmacodynamics
The AUC/minimum inhibitory concentration (MIC) was shown to best correlate with tedizolid activity in animal infection models.
In the mouse thigh infection model of S. aureus, antistaphylococcal killing activity was impacted by the presence of granulocytes. In granulocytopenic mice (neutrophil count <100 cells/mL), bacterial stasis was achieved at a human-equivalent dose of approximately 2000 mg/day; whereas, in non-granulocytopenic animals, stasis was achieved at a human-equivalent dose of approximately 100 mg/day. The safety and efficacy of SIVEXTRO for the treatment of neutropenic patients (neutrophil counts <1000 cells/mm3) have not been evaluated.
12.3 Pharmacokinetics
Tedizolid phosphate is a prodrug that is converted by phosphatases to tedizolid, the microbiologically active moiety, following oral and intravenous administration. Only the pharmacokinetic profile of tedizolid is discussed further due to negligible systemic exposure of tedizolid phosphate following oral and intravenous administration. Following multiple once-daily oral or intravenous administration, steady state concentrations are achieved within approximately three days with tedizolid accumulation of approximately 30% (tedizolid half-life of approximately 12 hours). Pharmacokinetic (PK) parameters of tedizolid following oral and intravenous administration of 200 mg once daily tedizolid phosphate are shown in Table 5.
| Pharmacokinetic Parameters of Tedizolid* | Oral | Intravenous | ||
|---|---|---|---|---|
| Single Dose | Steady State | Single Dose | Steady State | |
|
||||
| Cmax (mcg/mL) | 2.0 (0.7) | 2.2 (0.6) | 2.3 (0.6) | 3.0 (0.7) |
| Tmax (hr)† | 2.5 (1.0 - 8.0) | 3.5 (1.0 - 6.0) | 1.1 (0.9 - 1.5) | 1.2 (0.9 - 1.5) |
| AUC (mcg∙hr/mL)‡ | 23.8 (6.8) | 25.6 (8.5) | 26.6 (5.2) | 29.2 (6.2) |
| CL or CL/F (L/hr) | 7.5 (2.3) | 6.9 (1.7) | 6.4 (1.2) | 5.9 (1.4) |
13. Nonclinical Toxicology
13.2 Animal Toxicology and/or Pharmacology
Repeated-oral and intravenous dosing of tedizolid phosphate in rats in 1-month and 3-month toxicology studies produced dose- and time-dependent bone marrow hypocellularity (myeloid, erythroid, and megakaryocyte), with associated reduction in circulating RBCs, WBCs, and platelets. These effects showed evidence of reversibility and occurred at plasma tedizolid exposure levels (AUC) ≥6-fold greater than the plasma exposure associated with the human therapeutic dose. In a 1-month immunotoxicology study in rats, repeated oral dosing of tedizolid phosphate was shown to significantly reduce splenic B cells and T cells and reduce plasma IgG titers. These effects occurred at plasma tedizolid exposure levels (AUC) ≥3-fold greater than the expected human plasma exposure associated with the therapeutic dose.
14. Clinical Studies
14.1 Acute Bacterial Skin and Skin Structure Infections
Adults
A total of 1333 adults with acute bacterial skin and skin structure infections (ABSSSI) were randomized in two multicenter, multinational, double-blind, non-inferiority trials. Both trials compared SIVEXTRO 200 mg once daily for 6 days versus linezolid 600 mg every 12 hours for 10 days. In Trial 1, patients were treated with oral therapy, while in Trial 2, patients could receive oral therapy after a minimum of one day of intravenous therapy. Patients with cellulitis/erysipelas, major cutaneous abscess, or wound infection were enrolled in the trials. Patients with wound infections could have received aztreonam and/or metronidazole as adjunctive therapy for gram-negative bacterial coverage, if needed. The intent-to-treat (ITT) patient population included all randomized patients.
In Trial 1, 332 patients with ABSSSI were randomized to SIVEXTRO and 335 patients were randomized to linezolid. The majority (91%) of patients treated with SIVEXTRO in Trial 1 were less than 65 years old with a median age of 43 years (range: 18 to 86 years). Patients treated with SIVEXTRO were predominantly male (61%) and White (84%); 13% had BMI ≥35 kg/m2, 8% had diabetes mellitus, 35% were current or recent intravenous drug users, and 2% had moderate to severe renal impairment. The overall median surface area of infection was 188 cm2. The types of ABSSSI included were cellulitis/erysipelas (41%), wound infection (29%), and major cutaneous abscess (30%). In addition to local signs and symptoms of infection, patients were also required to have at least one regional or systemic sign of infection at baseline, defined as lymphadenopathy (87% of patients), temperature 38°C or higher (16% of patients), white blood cell count greater than 10,000 cells/mm3 or less than 4000 cells/mm3 (42%), or 10% or more band forms on white blood cell differential (4%).
The primary endpoint in Trial 1 was early clinical response defined as no increase from baseline lesion area at 48-72 hours after the first dose and oral temperature of ≤37.6°C, confirmed by a second temperature measurement within 24 hours in the ITT population.
In Trial 2, 332 patients with ABSSSI were randomized to SIVEXTRO and 334 patients were randomized to linezolid. The majority (87%) of patients treated with SIVEXTRO in Trial 2 were less than 65 years old with a median age of 46 years (range: 17 to 86 years). Patients treated with SIVEXTRO were predominantly male (68%) and White (86%); 16% had BMI ≥35 kg/m2, 10% had diabetes mellitus, 20% were current or recent intravenous drug users, and 4% had moderate to severe renal impairment. The overall median surface area of infection was 231 cm2. The types of ABSSSI included were cellulitis/erysipelas (50%), wound infection (30%), and major cutaneous abscess (20%). In addition to local signs and symptoms of infection, patients were also required to have at least one regional or systemic sign of infection at baseline, defined as lymphadenopathy (71% of patients), temperature 38°C or higher (31% of patients), white blood cell count greater than 10,000 cells/mm3 or less than 4000 cells/mm3 (53%), or 10% or more band forms on white blood cell differential (16%).
The primary endpoint in Trial 2 was early clinical response defined as at least a 20% decrease from baseline lesion area at 48-72 hours after the first dose in the ITT population (Table 6).
| SIVEXTRO (200 mg) | Linezolid (1200 mg) | Treatment Difference (2-sided 95% CI) |
|
|---|---|---|---|
| CI=confidence interval | |||
|
|||
| No increase in lesion surface area from baseline and oral temperature of ≤37.6°C, confirmed by a second temperature measurement within 24 hours at 48-72 hours* | |||
| Trial 1, N | 332 | 335 | |
| Responder, n (%) | 264 (79.5) | 266 (79.4) | 0.1 (-6.1, 6.2) |
| Trial 2, N | 332 | 334 | |
| Responder, n (%) | 286 (86.1) | 281 (84.1) | 2.0 (-3.5, 7.3) |
| At least a 20% decrease from baseline in lesion area at 48-72 hours† | |||
| Trial 1, N | 332 | 335 | |
| Responder, n (%) | 259 (78.0) | 255 (76.1) | 1.9 (-4.5, 8.3) |
| Trial 2, N | 332 | 334 | |
| Responder, n (%) | 283 (85.2) | 276 (82.6) | 2.6 (-3.0, 8.2) |
An investigator assessment of clinical response was made at the post-therapy evaluation (PTE) (7 - 14 days after the end of therapy) in the ITT and CE (Clinically Evaluable) populations. Clinical success was defined as resolution or near resolution of most disease-specific signs and symptoms, absence or near resolution of systemic signs of infection if present at baseline (lymphadenopathy, fever, >10% immature neutrophils, abnormal WBC count), and no new signs, symptoms, or complications attributable to the ABSSSI requiring further treatment of the primary lesion (Table 7).
| SIVEXTRO (200 mg) n/N (%) | Linezolid (1200 mg) n/N (%) | Treatment Difference (2-sided 95% CI) |
|
|---|---|---|---|
| CI=confidence interval; ITT=intent-to-treat; CE=clinically evaluable | |||
| Trial 1 | |||
| ITT | 284/332 (85.5) | 288/335 (86.0) | -0.5 (-5.8, 4.9) |
| CE | 264/279 (94.6) | 267/280 (95.4) | -0.8 (-4.6, 3.0) |
| Trial 2 | |||
| ITT | 292/332 (88.0) | 293/334 (87.7) | 0.3 (-4.8, 5.3) |
| CE | 268/290 (92.4) | 269/280 (96.1) | -3.7 (-7.7, 0.2) |
Clinical success by baseline pathogens from the primary infection site or blood cultures for the microbiological intent-to-treat (MITT) patient population for two integrated Phase 3 ABSSSI studies are presented in Table 8 and Table 9.
| Pathogen | No increase in lesion surface area from baseline and oral temperature of ≤37.6°C* | At least a 20% decrease from baseline in lesion area† | ||
|---|---|---|---|---|
| SIVEXTRO (200 mg) n/N (%) | Linezolid (1200 mg) n/N (%) | SIVEXTRO (200 mg) n/N (%) | Linezolid (1200 mg) n/N (%) |
|
| Pooled analysis; n=number of patients in the specific category; N=Number of patients with the specific pathogen isolated from the ABSSSI | ||||
|
||||
| Staphylococcus aureus | 276/329 (83.9) | 278/342 (81.3) | 280/329 (85.1) | 276/342 (80.7) |
| Methicillin-resistant S. aureus | 112/141 (79.4) | 113/146 (77.4) | 114/141 (80.9) | 111/146 (76.0) |
| Methicillin-susceptible S. aureus | 164/188 (87.2) | 167/198 (84.3) | 166/188 (88.3) | 167/198 (84.3) |
| Streptococcus pyogenes | 27/33 (81.8) | 18/20 (90.0) | 25/33 (75.8) | 16/20 (80.0) |
| Streptococcus anginosus Group | 22/30 (73.3) | 26/28 (92.9) | 22/30 (73.3) | 25/28 (89.3) |
| Streptococcus agalactiae | 6/9 (66.7) | 8/10 (80.0) | 6/9 (66.7) | 7/10 (70.0) |
| Enterococcus faecalis | 7/10 (70.0) | 3/4 (75.0) | 6/10 (60.0) | 1/4 (25.0) |
Baseline bacteremia in the tedizolid arm with relevant pathogens included two subjects with MRSA, four subjects with MSSA, two subjects with S. pyogenes, one subject with S. agalactiae, and one subject with S. constellatus. All of these subjects were Responders at the 48-72 hour evaluation. At the Post-therapy Evaluation (PTE), 8 of 10 subjects were considered clinical successes.
| Pathogen | Clinical Response at PTE | |
|---|---|---|
| SIVEXTRO (200 mg) n/N (%) | Linezolid (1200 mg) n/N (%) |
|
| Pooled analysis; n=number of patients in the specific category; N=Number of patients with the specific pathogen isolated from the ABSSSI | ||
| Staphylococcus aureus | 291/329 (88.5) | 303/342 (88.6) |
| Methicillin-resistant S. aureus | 118/141 (83.7) | 119/146 (81.5) |
| Methicillin-susceptible S. aureus | 173/188 (92.0) | 186/198 (93.9) |
| Streptococcus pyogenes | 30/33 (90.9) | 19/20 (95.0) |
| Streptococcus anginosus Group | 21/30 (70.0) | 25/28 (89.3) |
| Streptococcus agalactiae | 8/9 (88.9) | 8/10 (80.0) |
| Enterococcus faecalis | 7/10 (70.0) | 4/4 (100.0) |
Baseline bacteremia in the tedizolid arm with relevant pathogens included two subjects with MRSA, four subjects with MSSA, two subjects with S. pyogenes, one subject with S. agalactiae, and one subject with S. constellatus. All of these subjects were Responders at the 48-72 hour evaluation. At the Post-therapy Evaluation (PTE) 8 of 10 subjects were considered clinical successes.
Pediatric Patients
The safety and efficacy of SIVEXTRO in pediatric patients 12 to < 18 years of age were investigated in a randomized, single blind, active-controlled trial of 120 patients with clinically documented ABSSSI (91 receiving tedizolid, 29 receiving comparator). Patients were randomized in a 3:1 ratio with stratification by geographic region to receive SIVEXTRO IV and/or oral therapy, dosed 200 mg once daily for 6 days, or comparator IV and/or oral therapy, dosed over 10 days. Comparator therapy was selected by the investigator from a list of 5 IV and 4 oral comparators per local standard of care. The most frequently used comparators were cefazolin (11 patients) and vancomycin (8 patients).
The primary objective was to evaluate the safety and tolerability of SIVEXTRO. The trial was not powered for comparative inferential efficacy analysis. Clinical response at the test of cure visit (Day 18-25) was assessed by a blinded investigator in the ITT population (all randomized patients). Clinical successes were required to have resolution or near resolution of all related signs and symptoms such that no further antibacterial therapy was needed. Early clinical response, defined as at least a 20% reduction in lesion size at 48-72 hours after start of treatment, was also assessed in the ITT population.
Clinical success at test of cure was 96.7% (88/91) in the tedizolid group and 93.1% (27/29) in the comparator group (difference 3.6%, 95% CI: -6.3, 13.5). Early clinical response at 48-72 hours was 92.3% (84/91) in the tedizolid group and 96.6% (28/29) in the comparator group (difference -4.2%, 95% CI: -12.9, 4.4).
16. How is Sivextro supplied
16.1 Tablets
SIVEXTRO tablets are yellow film-coated oval tablets containing 200 mg of tedizolid phosphate; each tablet is debossed with "TZD" on one side and "200" on the other side.
They are supplied as follows:
HDPE bottles of 30 tablets with child-resistant closure (NDC 72000-310-30)
Unit dose blister packs of 6 tablets (NDC 72000-310-06)
16.2 For Injection
SIVEXTRO is supplied as a sterile, lyophilized powder for injection in single-dose vials of 200 mg. Each 200 mg vial must be reconstituted with Sterile Water for Injection and subsequently diluted only with 0.9% Sodium Chloride Injection, USP.
They are supplied as follows:
Package of ten 200 mg single-dose vials (NDC 72000-320-10)
| SIVEXTRO
tedizolid phosphate tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| SIVEXTRO
tedizolid phosphate injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Nabriva Therapeutics US, Inc. (072863844) |
| Registrant - Merck Sharp & Dohme LLC (118446553) |