Drug Detail:Sorilux (Calcipotriene topical [ cal-sih-poh-try-een ])
Drug Class: Topical antipsoriatics
Highlights of Prescribing Information
SORILUX (calcipotriene) foam, for topical use
Initial U.S. Approval: 1993
Recent Major Changes
| Indications and Usage (1) | 11/2019 |
Indications and Usage for Sorilux
SORILUX® Foam is a vitamin D analog indicated for the topical treatment of plaque psoriasis of the scalp and body in adults and pediatric patients 4 years of age and older. (1)
Sorilux Dosage and Administration
- For topical use only; not for oral, ophthalmic, or intravaginal use. (2)
- Apply twice daily. (2)
Dosage Forms and Strengths
0.005%, foam. (3)
Contraindications
- Do not use in patients with known hypercalcemia. (4)
Warnings and Precautions
- Flammability: Contents are flammable. Instruct the patient the avoid fire, flame, and smoking during and immediately following application. (5.1)
- Effects on Calcium Metabolism: If elevation of serum calcium occurs, instruct patients to discontinue treatment until normal calcium levels are restored. (5.2)
Adverse Reactions/Side Effects
Adverse reactions reported in ≥ 1% of subjects treated with SORILUX Foam and at a higher incidence than subjects treated with vehicle were application site erythema and application site pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Mayne Pharma at 1-844-825-8500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2019
Related/similar drugs
Cosentyx, Otezla, Stelara, Sotyktu, methotrexate, Humira, EnbrelFull Prescribing Information
1. Indications and Usage for Sorilux
SORILUX Foam is indicated for the topical treatment of plaque psoriasis of the scalp and body in adults and pediatric patients 4 years of age and older.
2. Sorilux Dosage and Administration
SORILUX Foam is for topical use only. SORILUX Foam is not for oral, ophthalmic, or intravaginal use.
Apply a thin layer of SORILUX Foam twice daily to the affected areas and rub in gently and completely. Avoid contact with the face and eyes.
3. Dosage Forms and Strengths
0.005%, white foam. SORILUX Foam contains calcipotriene 50 mcg/g in an aqueous-based emulsion foam vehicle.
5. Warnings and Precautions
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
SORILUX Foam was studied in four vehicle-controlled trials. A total of 1094 adult subjects with plaque psoriasis, including 654 exposed to SORILUX Foam, were treated twice daily for 8 weeks. Adverse reactions reported in ≥1% of subjects treated with SORILUX Foam and at a higher incidence than subjects treated with vehicle were application site erythema (2%) and application site pain (3%). The incidence of these adverse reactions was similar between the body and scalp.
In an open-label study, 19 pediatric subjects age 12 to less than 17 years applied SORILUX Foam twice daily for 14 days and once on Day 15. Adverse reactions included application site pain, application site pruritus and pruritus [see Clinical Pharmacology (12.2 and 12.3) and Pediatric Use (8.4)].
In an open-label study, 36 pediatric subjects age 4 to less than 12 years applied SORILUX Foam twice daily for up to 8 weeks. Adverse reactions included application site pain and contact dermatitis [see Clinical Pharmacology (12.2 and 12.3) and Pediatric Use (8.4)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of SORILUX Foam. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and Subcutaneous: application site vesicles
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of SORILUX Foam have been established in pediatric patients age 4 years and older for topical treatment of plaque psoriasis of the scalp and body.
Use of SORILUX Foam in this age group is supported by two adequate and well controlled 8-week trials in adults and adolescents 12 years of age and older, with additional data from a 15-day open-label safety and pharmacokinetics (PK) study conducted in 19 subjects 12 to less than 17 years of age; and an 8-week open-label safety and PK study in 36 subjects 4 to 11 years of age with psoriasis. Data from 19 subjects aged 12 to less than 17 years and 18 subjects aged 5 to 11 years showed no significant effects on indices of calcium metabolism. Systemic concentrations of calcipotriene were not quantifiable in the two studies in subjects aged 7 years to less than 17 years. [see Clinical Studies (14), Clinical Pharmacology (12.2, 12.3) and Adverse Reactions (6.1)].
The safety and effectiveness of SORILUX Foam in pediatric patients less than 4 years of age have not been established.
8.5 Geriatric Use
Clinical trials of SORILUX Foam did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
10. Overdosage
Topically applied calcipotriene can be absorbed in sufficient amounts to produce systemic effects. Elevated serum calcium has been observed with use of topical calcipotriene [See Warnings and Precautions (5.2)].
11. Sorilux Description
SORILUX Foam contains the compound calcipotriene, a synthetic vitamin D3 analog, in an aqueous-based emulsion foam vehicle for topical dermatologic use.
Chemically, calcipotriene is (5Z,7E,22E,24S)-24-cyclopropyl-9,10-secochola-5,7,10(19), 22-tetraene- 1α,3β,24-triol. The structural formula is represented below:
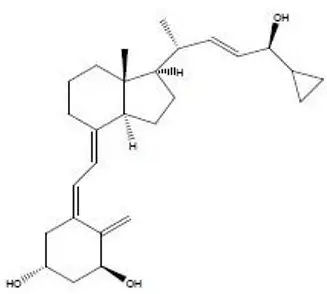
Molecular Formula: C27H40O3 Molecular Weight: 412.6
Calcipotriene is a white or off-white crystalline substance. SORILUX Foam contains calcipotriene 50 mcg/g in an aqueous-based emulsion foam vehicle consisting of cetyl alcohol, dibasic sodium phosphate, dl-α-tocopherol, edetate disodium, isopropyl myristate, light mineral oil, polyoxyl 20 cetostearyl ether, propylene glycol, purified water, stearyl alcohol, and white petrolatum. SORILUX Foam is dispensed from an aluminum can pressurized with a hydrocarbon (propane/n-butane/isobutane) propellant.
12. Sorilux - Clinical Pharmacology
12.1 Mechanism of Action
Calcipotriene is a synthetic vitamin D3 analog that has a similar receptor binding affinity as natural vitamin D3. However, the exact mechanism of action contributing to the clinical efficacy in the treatment of psoriasis is unknown.
12.3 Pharmacokinetics
The systemic absorption of calcipotriene in subjects with psoriasis of the body was evaluated at steady state following application of either SORILUX Foam or calcipotriene ointment to a body surface area of 5% to 10%. In the SORILUX Foam treatment group, 15 out of 16 subjects had calcipotriene plasma concentrations below the limit of quantitation (10 pg/mL), while in the calcipotriene ointment treated group, 5 out of 16 subjects had measurable calcipotriene plasma concentrations at various time points. All measurable plasma calcipotriene concentrations were below 25 pg/mL.
The pharmacokinetics of SORILUX Foam, 0.005% was investigated when applied topically for 15 days to 17 subjects aged 12 to less than 17 years with moderate plaque psoriasis involving a mean BSA of 24%, excluding face and scalp, and a mean scalp involvement of 43%. Systemic concentration of calcipotriene were not quantifiable in any of the subjects (limit of quantification = 10 pg/mL).
In 11 pediatric subjects 7 to 11 years of age with plaque psoriasis involving a mean BSA of 10%, plasma concentration of calcipotriene was measured following 2 weeks of twice daily administration of SORILUX Foam. No subject had quantifiable calcipotriene plasma concentration (limit of quantification = 10 pg/mL).
The systemic disposition of calcipotriene is expected to be similar to that of the naturally occurring vitamin D. Absorbed calcipotriene is known to be converted to inactive metabolites within 24 hours of application and the metabolism occurs via a similar pathway to the natural hormone.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Calcipotriene topically administered to mice for up to 24 months at dose levels of 3, 10, or 30 mcg/kg/day (corresponding to 9, 30, or 90 mcg /m2/day) showed no significant changes in tumor incidence when compared with controls.
The genotoxic potential of calcipotriene was evaluated in an Ames assay, a mouse lymphoma TK locus assay, a human lymphocyte chromosome aberration assay, and a mouse micronucleus assay. All assay results were negative.
Studies in rats at oral doses up to 54 mcg /kg/day (318 mcg /m2/day) of calcipotriene indicated no impairment of fertility or general reproductive performance.
14. Clinical Studies
In two multi-center, randomized, double-blind, vehicle-controlled clinical trials a total of 659 subjects with psoriasis were randomized 2:1 to SORILUX Foam or vehicle; subjects applied the assigned treatment twice daily for 8 weeks. Baseline disease severity was graded using a 5-point Investigator Static Global Assessment scale (ISGA), on which subjects scored either "mild" or "moderate" as shown in Table 1.
| Disease Severity | Grade | Definition |
|---|---|---|
| Clear | 0 | No evidence of scaling, erythema, or plaque thickness |
| Almost clear | 1 | Occasional fine scale, faint erythema, and barely perceptible plaque thickness |
| Mild | 2 | Fine scale with light coloration and mild plaque elevation |
| Moderate | 3 | Coarse scale with moderate red coloration and moderate plaque thickness |
| Severe | 4 | Thick tenacious scale with deep coloration and severe plaque thickness |
Efficacy evaluation was carried out at Week 8 with treatment success being defined as a score of "clear" (grade 0) or "almost clear" (grade 1) and at least 2 grade improvement from the baseline score. Approximately 30% of enrolled subjects were graded as "mild" on the ISGA scale. The study population ranged in age from 12 to 89 years with 10 subjects less than 18 years of age at baseline. The subjects were 54% male and 88% Caucasian. Table 2 presents the efficacy results for each trial.
| Trial 1 | Trial 2 | |||
|---|---|---|---|---|
| SORILUX Foam N = 223 | Vehicle Foam N = 113 | SORILUX Foam N = 214 | Vehicle Foam N = 109 |
|
| Number (%) of Subjects with Treatment Success | 31 (14%) | 8 (7%) | 58 (27%) | 17 (16%) |
In one trial, subjects graded as "mild" at baseline showed a greater response to vehicle than SORILUX Foam.
Table 3 presents the success rates by disease severity at baseline for each trial.
| Trial 1 | Trial 2 | |||
|---|---|---|---|---|
| ISGA Scores at Baseline | SORILUX Foam (N = 223) | Vehicle Foam (N = 113) | SORILUX Foam (N = 214) | Vehicle Foam (N = 109) |
| Mild | 2/73 (2.7%) | 3/34 (8.8%) | 8/56 (14.3%) | 4/31 (12.9%) |
| Moderate | 29/150 (19.3%) | 5/79 (6.3%) | 50/158 (31.6%) | 13/78 (16.7%) |
In another multi-center, randomized, double-blind, vehicle-controlled clinical trial, a total of 363 subjects with moderate plaque psoriasis of the scalp and body were randomized 1:1 to SORILUX Foam or vehicle. Subjects applied the assigned treatment to the affected areas twice daily for 8 weeks.
Baseline disease severity of the scalp was graded using a 6-point ISGA; a score of "moderate" corresponded to grade 3.
The primary efficacy evaluation for scalp involvement was carried out at Week 8 with treatment success being defined as a score of "clear" (grade 0) or "almost clear" (grade 1). The study population ranged in age from 12 to 97 years with 11 subjects less than 18 years of age at baseline. The subjects were 60% male and 87% Caucasian. Table 4 presents the efficacy results for the trial.
| Trial 3 | ||
|---|---|---|
| SORILUX Foam N = 181 | Vehicle Foam N = 182 |
|
| Number (%) of Subjects with Treatment Success | 74 (41%) | 44 (24%) |
The contribution to efficacy of individual components of the vehicle has not been established.
16. How is Sorilux supplied
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Inform the patient to adhere to the following instructions:
- Apply SORILUX Foam to the affected skin areas.
- Apply SORILUX Foam to the scalp when the hair is dry.
- Avoid fire, flame, and smoking during and immediately following application since SORILUX Foam is flammable.
- Avoid contact with the face and eyes. If SORILUX Foam gets on the face or in or near their eyes, rinse thoroughly with water.
- Talk to your doctor if your skin does not improve after treatment with SORILUX Foam for 8 weeks.
- Wash your hands after applying SORILUX Foam unless your hands are the affected site.
- Do not place SORILUX Foam in the refrigerator or freezer.
Instructions for UseSORILUX (SOR-i-lux)(calcipotriene)Foam, 0.005%
Read this Instructions for Use before you start using SORILUX Foam and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
Important Information:
- SORILUX Foam is for use on the skin only (topical use). Do not use SORILUX Foam in your mouth, eyes, or vagina.
- Avoid getting SORILUX Foam on your face or in your eyes. If you accidentally get SORILUX Foam on your face or in your eyes, rinse well with water.
How to apply SORILUX Foam to your body:
Follow your healthcare providers instructions on how much SORILUX Foam to use and where to use it. Wash your hands after applying SORILUX Foam unless you are treating areas on your hands.
Step 1. Before applying SORILUX Foam for the first time, break the tiny plastic piece at the base of the can's rim by gently pushing back (away from the piece) on the nozzle. See Figure A.
Step 2: Shake the can of SORILUX Foam before use. See Figure B.
Step 3: Turn the can of SORILUX Foam upside down and press the nozzle. See Figure C.
Step 4. Dispense a small amount of SORILUX Foam into the palm of your hand. See Figure D.
Step 5. Use enough SORILUX Foam to cover the affected area with a thin layer. Gently rub the foam into the affected area until it disappears into the skin. See Figures E and F
How to apply SORILUX Foam to your scalp:
Step 6. Apply SORILUX Foam to your scalp when your hair is dry. Part your hair and apply directly on the affected area. See Figure G. Wash your hands after applying SORILUX Foam.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by: Mayne Pharma, Greenville, NC 27834
Revised: 11/2019
| SORILUX
calcipotriene aerosol, foam |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Mayne Pharma (867220261) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DPT Laboratories, Ltd. | 832224526 | ANALYSIS(51862-376) , MANUFACTURE(51862-376) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DPT Laboratories, Ltd. | 832224591 | PACK(51862-376) , LABEL(51862-376) | |




 Figure A
Figure A Figure B
Figure B Figure C
Figure C Figure D
Figure D Figure E
Figure E Figure F
Figure F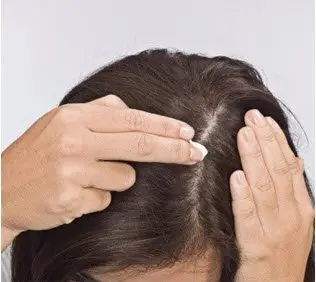 Figure G
Figure G