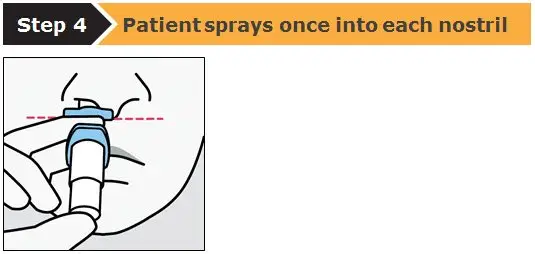
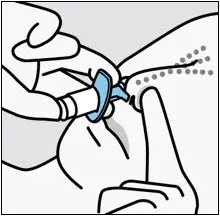
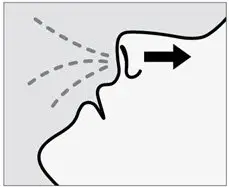
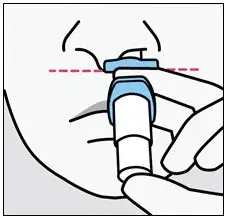
Drug Detail:Spravato nasal spray (Esketamine (nasal) [ es-ket-a-meen ])
Drug Class: Miscellaneous antidepressants
Highlights of Prescribing Information
SPRAVATO ® (esketamine) nasal spray, CIII
Initial U.S. Approval: 1970 (ketamine)
WARNING: SEDATION; DISSOCIATION; ABUSE AND MISUSE; and SUICIDAL THOUGHTS AND BEHAVIORS
See full prescribing information for complete boxed warning.
- Risk for sedation and dissociation after administration. Monitor patients for at least two hours after administration. ( 5.1, 5.2)
- Potential for abuse and misuse. Consider the risks and benefits of prescribing SPRAVATO prior to using in patients at higher risk of abuse. Monitor patients for signs and symptoms of abuse and misuse. ( 5.3)
- SPRAVATO is only available through a restricted program called the SPRAVATO REMS. ( 5.4)
- Increased risk of suicidal thoughts and behaviors in pediatric and young adult patients taking antidepressants. Closely monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors. SPRAVATO is not approved for use in pediatric patients. ( 5.5)
Recent Major Changes
| Indications and Usage ( 1) | 07/2020 |
| Dosage and Administration ( 2.3, 2.6) | 07/2020 |
| Warnings and Precautions ( 5.1, 5.2, 5.4, 5.6) | 07/2020 |
Indications and Usage for Spravato
SPRAVATO ® is a non-competitive N-methyl D-aspartate (NMDA) receptor antagonist indicated, in conjunction with an oral antidepressant, for the treatment of:
- Treatment-resistant depression (TRD) in adults. ( 1)
- Depressive symptoms in adults with major depressive disorder (MDD) with acute suicidal ideation or behavior. ( 1)
Limitations of Use:
- The effectiveness of SPRAVATO in preventing suicide or in reducing suicidal ideation or behavior has not been demonstrated. Use of SPRAVATO does not preclude the need for hospitalization if clinically warranted, even if patients experience improvement after an initial dose of SPRAVATO. ( 1)
- SPRAVATO is not approved as an anesthetic agent. The safety and effectiveness of SPRAVATO as an anesthetic agent have not been established. ( 1)
Spravato Dosage and Administration
- Administer SPRAVATO intranasally under the supervision of a healthcare provider. ( 2.1)
- Assess blood pressure prior to and after administration. ( 2.1)
- TRD: Evidence of therapeutic benefit should be evaluated at the end of the 4-week induction phase to determine need for continued treatment. ( 2.2)
- Depressive symptoms in MDD with acute suicidal ideation or behavior: Evidence of therapeutic benefit should be evaluated after 4 weeks to determine need for continued treatment. Treatment beyond 4 weeks has not been systematically evaluated. ( 2.3)
- See Full Prescribing Information for recommended dosage. ( 2.2, 2.3)
- See Full Prescribing Information for important administration instructions. ( 2.4)
Dosage Forms and Strengths
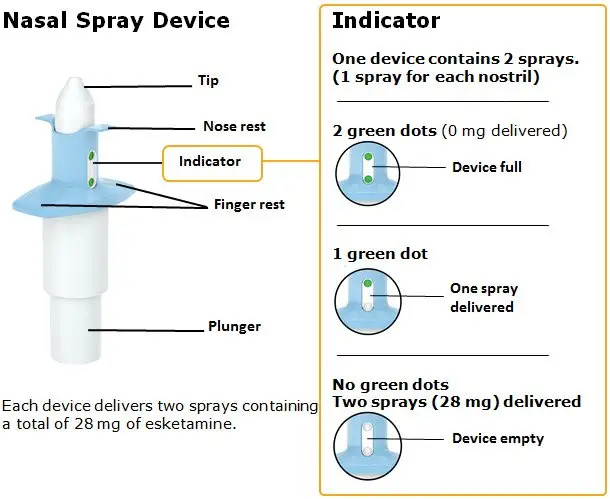
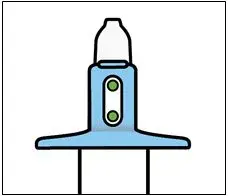
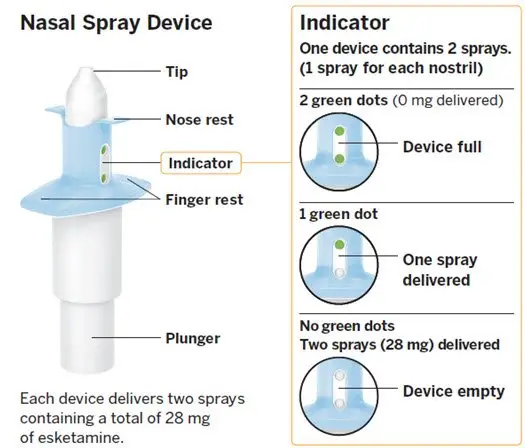
Nasal Spray: 28 mg of esketamine per device. Each nasal spray device delivers two sprays containing a total of 28 mg of esketamine. ( 3)
Contraindications
- Aneurysmal vascular disease (including thoracic and abdominal aorta, intracranial and peripheral arterial vessels) or arteriovenous malformation. ( 4)
- Intracerebral hemorrhage. ( 4)
- Hypersensitivity to esketamine, ketamine, or any of the excipients. ( 4)
Warnings and Precautions
- Increases in Blood Pressure: Patients with cardiovascular and cerebrovascular conditions and risk factors may be at an increased risk of associated adverse effects. ( 5.6)
- Cognitive Impairment: SPRAVATO may impair attention, judgment, thinking, reaction speed and motor skills. ( 5.7)
- Impaired Ability to Drive and Operate Machinery: Do not drive or operate machinery until the next day after a restful sleep. ( 5.8)
- Embryo-fetal Toxicity: May cause fetal harm. Consider pregnancy planning and prevention in females of reproductive potential. ( 5.10, 8.1, 8.3)
Adverse Reactions/Side Effects
The most commonly observed adverse reactions (incidence ≥5% and at least twice that of placebo plus oral antidepressant):
- TRD: dissociation, dizziness, nausea, sedation, vertigo, hypoesthesia, anxiety, lethargy, blood pressure increased, vomiting, and feeling drunk. ( 6)
- Treatment of depressive symptoms in adults with MDD with acute suicidal ideation or behavior: dissociation, dizziness, sedation, blood pressure increased, hypoesthesia, vomiting, euphoric mood, and vertigo. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Pharmaceuticals, Inc. at 1-800-JANSSEN (1-800-526-7736) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
- Lactation: Breastfeeding not recommended. ( 8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 7/2020
Full Prescribing Information
1. Indications and Usage for Spravato
SPRAVATO ® is indicated, in conjunction with an oral antidepressant, for the treatment of:
- Treatment-resistant depression (TRD) in adults
- Depressive symptoms in adults with major depressive disorder (MDD) with acute suicidal ideation or behavior
2. Spravato Dosage and Administration
2.1 Important Considerations Prior to Initiating and During Therapy
SPRAVATO must be administered under the direct supervision of a healthcare provider. A treatment session consists of nasal administration of SPRAVATO and post-administration observation under supervision.
2.2 Treatment-Resistant Depression
Administer SPRAVATO in conjunction with an oral antidepressant (AD).
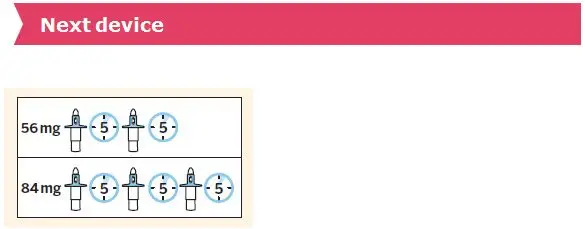
The recommended dosage of SPRAVATO for the treatment of TRD in adults is shown in Table 1. Dosage adjustments should be made based on efficacy and tolerability. Evidence of therapeutic benefit should be evaluated at the end of the induction phase to determine need for continued treatment.
|
||
| Adults | ||
| Induction Phase | Weeks 1 to 4: | Day 1 starting dose: 56 mg |
| Administer twice per week | Subsequent doses: 56 mg or 84 mg | |
| Maintenance Phase | Weeks 5 to 8: | |
| Administer once weekly | 56 mg or 84 mg | |
| Week 9 and after: | ||
| Administer every 2 weeks or once weekly * | 56 mg or 84 mg | |
2.3 Depressive Symptoms in Patients with Major Depressive Disorder with Acute Suicidal Ideation or Behavior
Administer SPRAVATO in conjunction with an oral antidepressant (AD).
The recommended dosage of SPRAVATO for the treatment of depressive symptoms in adults with MDD with acute suicidal ideation or behavior is 84 mg twice per week for 4 weeks. Dosage may be reduced to 56 mg twice per week based on tolerability. After 4 weeks of treatment with SPRAVATO, evidence of therapeutic benefit should be evaluated to determine need for continued treatment. The use of SPRAVATO, in conjunction with an oral antidepressant, beyond 4 weeks has not been systematically evaluated in the treatment of depressive symptoms in patients with MDD with acute suicidal ideation or behavior.
2.4 Administration Instructions
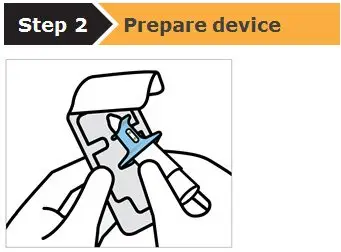
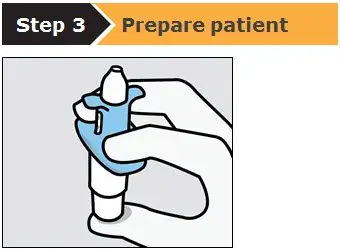
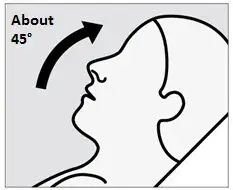
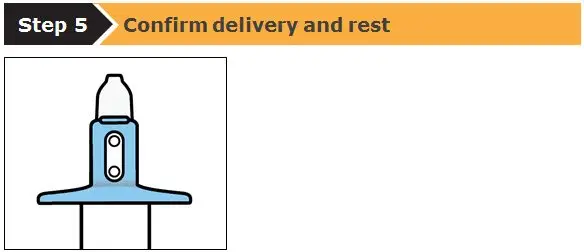
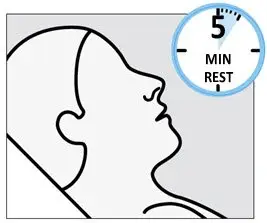
SPRAVATO is for nasal use only. The nasal spray device delivers a total of 28 mg of esketamine. To prevent loss of medication, do not prime the device before use. Use 2 devices (for a 56 mg dose) or 3 devices (for an 84 mg dose), with a 5-minute rest between use of each device. Follow these administration instructions and read the Instructions for Use before administration:
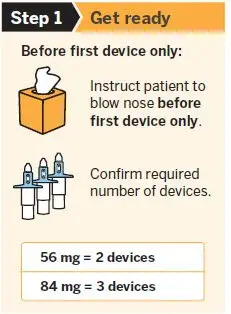
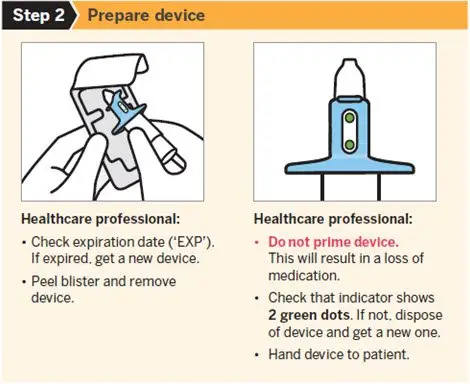
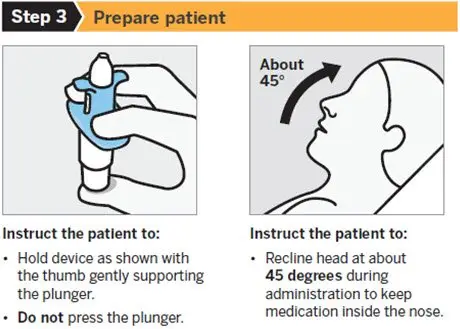

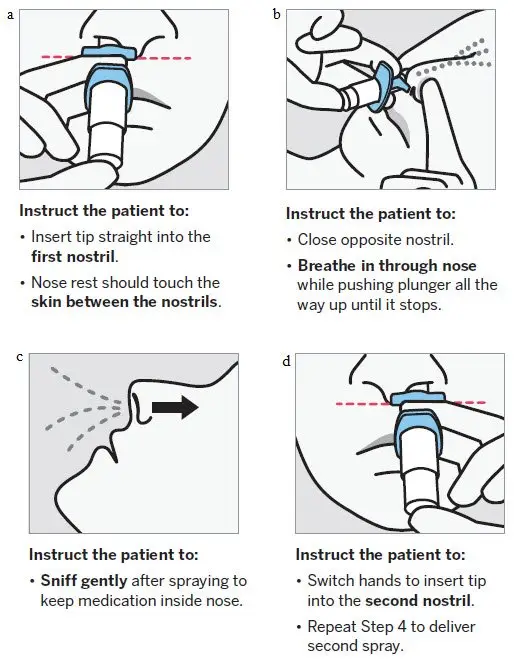
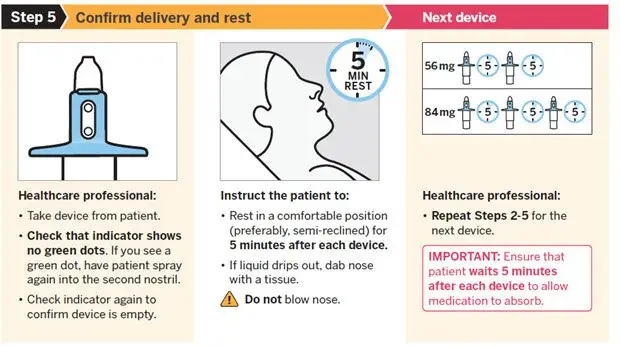

2.5 Post-Administration Observation
During and after SPRAVATO administration at each treatment session, observe the patient for at least 2 hours until the patient is safe to leave [see Warnings and Precautions (5.1, 5.2, 5.6, 5.8)] . Before SPRAVATO administration, instruct patients not to engage in potentially hazardous activities, such as driving a motor vehicle or operating machinery, until the next day after a restful sleep.
2.6 Missed Treatment Session(s)
If a patient misses treatment session(s), provided there is no worsening of their depressive symptoms, the patient should continue the current dosing schedule.
For patients who miss treatment session(s) during maintenance treatment and have worsening of depression symptoms, per clinical judgement, consider returning to the previous dosing schedule (e.g., if doses missed during weekly dosing, revert to twice weekly dosing).
3. Dosage Forms and Strengths
Nasal Spray: 28 mg of esketamine per device. Each nasal spray device delivers two sprays containing a total of 28 mg esketamine.
4. Contraindications
SPRAVATO is contraindicated in patients with:
- Aneurysmal vascular disease (including thoracic and abdominal aorta, intracranial, and peripheral arterial vessels) or arteriovenous malformation [see Warnings and Precautions (5.6)]
- History of intracerebral hemorrhage [see Warnings and Precautions (5.6)]
- Hypersensitivity to esketamine, ketamine, or any of the excipients.
5. Warnings and Precautions
5.1 Sedation
In clinical trials, 48% to 61% of SPRAVATO-treated patients developed sedation based on the Modified Observer's Assessment of Alertness/Sedation scale (MOAA/S) [see Adverse Reactions (6.1)] , and 0.3% to 0.4% of SPRAVATO-treated patients experienced loss of consciousness (MOAA/S score of 0).
Because of the possibility of delayed or prolonged sedation, patients must be monitored by a healthcare provider for at least 2 hours at each treatment session, followed by an assessment to determine when the patient is considered clinically stable and ready to leave the healthcare setting [see Dosage and Administration (2.4)] .
Closely monitor for sedation with concomitant use of SPRAVATO with CNS depressants [see Drug Interaction (7.1)] .
SPRAVATO is available only through a restricted program under a REMS [see Warnings and Precautions (5.4)] .
5.2 Dissociation
The most common psychological effects of SPRAVATO were dissociative or perceptual changes (including distortion of time, space and illusions), derealization and depersonalization (61% to 84% of SPRAVATO-treated patients developed dissociative or perceptual changes based on the Clinician-Administered Dissociative States Scale) [see Adverse Reactions (6.1)] . Given its potential to induce dissociative effects, carefully assess patients with psychosis before administering SPRAVATO; treatment should be initiated only if the benefit outweighs the risk.
Because of the risks of dissociation, patients must be monitored by a healthcare provider for at least 2 hours at each treatment session, followed by an assessment to determine when the patient is considered clinically stable and ready to leave the healthcare setting [see Dosage and Administration (2.4)] .
SPRAVATO is available only through a restricted program under a REMS [see Warnings and Precautions (5.4)] .
5.3 Abuse and Misuse
SPRAVATO contains esketamine, a Schedule III controlled substance (CIII), and may be subject to abuse and diversion. Assess each patient's risk for abuse or misuse prior to prescribing SPRAVATO and monitor all patients receiving SPRAVATO for the development of these behaviors or conditions, including drug-seeking behavior, while on therapy. Contact local state professional licensing board or state-controlled substances authority for information on how to prevent and detect abuse or diversion of SPRAVATO. Individuals with a history of drug abuse or dependence are at greater risk; therefore, use careful consideration prior to treatment of individuals with a history of substance use disorder and monitor for signs of abuse or dependence [see Drug Abuse and Dependence (9)] .
SPRAVATO is available only through a restricted program under a REMS [see Warnings and Precautions (5.4)] .
5.4 SPRAVATO Risk Evaluation and Mitigation Strategy (REMS)
SPRAVATO is available only through a restricted program under a REMS called the SPRAVATO REMS because of the risks of serious adverse outcomes from sedation, dissociation, and abuse and misuse [see Boxed Warning and Warnings and Precautions (5.1, 5.2, 5.3)].
Important requirements of the SPRAVATO REMS include the following:
- Healthcare settings must be certified in the program and ensure that SPRAVATO is:
- Only dispensed and administered in healthcare settings.
- Patients treated in outpatient settings (e.g. medical offices and clinics) must be enrolled in the program.
- Administered by patients under the direct observation of a healthcare provider and that patients are monitored by a healthcare provider for at least 2 hours after administration of SPRAVATO [see Dosage and Administration (2.4)].
- Pharmacies must be certified in the REMS and must only dispense SPRAVATO to healthcare settings that are certified in the program.
Further information, including a list of certified pharmacies is available at www.SPRAVATOrems.com or 1-855-382-6022.
5.5 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included approximately 77,000 adult patients and 4,500 pediatric patients (SPRAVATO is not approved in pediatric patients), the incidence of suicidal thoughts and behaviors in patients age 24 years and younger was greater than in placebo-treated patients. There was considerable variation in risk of suicidal thoughts and behaviors among drugs, but there was an increased risk identified in young patients for most drugs studied. There were differences in absolute risk of suicidal thoughts and behaviors across the different indications, with the highest incidence in patients with major depressive disorder (MDD). The drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1000 patients treated are provided in Table 2.
| Age Range (Years) | Drug-Placebo Difference in Number of Patients with Suicidal Thoughts or Behaviors per 1000 Patients Treated |
|---|---|
|
|
| Increases Compared to Placebo | |
| <18 | 14 additional patients |
| 18–24 | 5 additional patients |
| Decreases Compared to Placebo | |
| 25–64 | 1 fewer patient |
| ≥65 | 6 fewer patients |
It is unknown whether the risk of suicidal thoughts and behaviors in children, adolescents, and young adults extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance studies in adults with MDD that antidepressants delay the recurrence of depression and that depression itself is a risk factor for suicidal thoughts and behaviors.
Monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen, including possibly discontinuing SPRAVATO and/or the concomitant oral antidepressant, in patients whose depression is persistently worse, or who are experiencing emergent suicidal thoughts or behaviors.
5.6 Increase in Blood Pressure
SPRAVATO causes increases in systolic and/or diastolic blood pressure (BP) at all recommended doses. Increases in BP peak approximately 40 minutes after SPRAVATO administration and last approximately 4 hours [see Adverse Reactions (6.1)] .
Approximately 8% to 19% of SPRAVATO-treated patients and 1% to 4% of placebo-treated patients experienced an increase of greater than or equal to 40 mmHg in systolic BP and/or 25 mmHg in diastolic BP in the first 1.5 hours after administration at least once during the first 4 weeks of treatment. A substantial increase in blood pressure could occur after any dose administered even if smaller blood pressure effects were observed with previous administrations. SPRAVATO is contraindicated in patients for whom an increase in BP or intracranial pressure poses a serious risk (e.g., aneurysmal vascular disease, arteriovenous malformation, history of intracerebral hemorrhage) [see Contraindications (4)]. Before prescribing SPRAVATO, patients with other cardiovascular and cerebrovascular conditions should be carefully assessed to determine whether the potential benefits of SPRAVATO outweigh its risks.
Assess BP prior to administration of SPRAVATO. In patients whose BP is elevated prior to SPRAVATO administration (as a general guide: >140/90 mmHg) a decision to delay SPRAVATO therapy should take into account the balance of benefit and risk in individual patients.
BP should be monitored for at least 2 hours after SPRAVATO administration [see Dosage and Administration (2.1, 2.4)] . Measure blood pressure around 40 minutes post-dose and subsequently as clinically warranted until values decline. If BP remains high, promptly seek assistance from practitioners experienced in BP management. Refer patients experiencing symptoms of a hypertensive crisis (e.g., chest pain, shortness of breath) or hypertensive encephalopathy (e.g., sudden severe headache, visual disturbances, seizures, diminished consciousness or focal neurological deficits) immediately for emergency care.
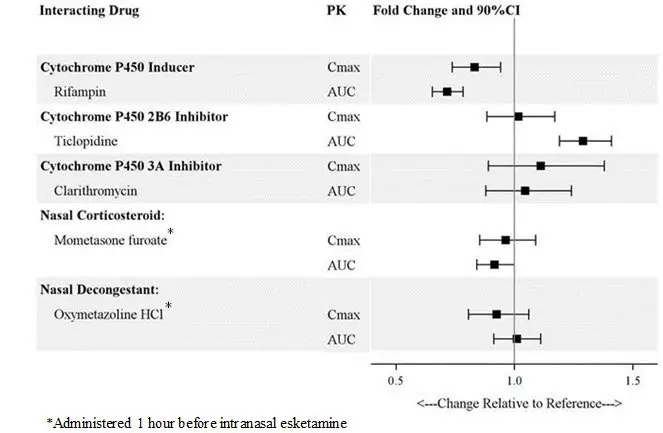
Closely monitor blood pressure with concomitant use of SPRAVATO with psychostimulants or monoamine oxidase inhibitors (MAOIs) [see Drug Interactions (7.2, 7.3)] .
In patients with history of hypertensive encephalopathy, more intensive monitoring, including more frequent blood pressure and symptom assessment, is warranted because these patients are at increased risk for developing encephalopathy with even small increases in blood pressure.
5.8 Impaired Ability to Drive and Operate Machinery
Two placebo-controlled studies were conducted to assess the effects of SPRAVATO on the ability to drive [see Clinical Studies (14.3)] . The effects of SPRAVATO 84 mg were comparable to placebo at 6 hours and 18 hours post-dose. However, two SPRAVATO-treated subjects in one of the studies discontinued the driving test at 8 hours post-dose because of SPRAVATO-related adverse reactions.
Before SPRAVATO administration, instruct patients not to engage in potentially hazardous activities requiring complete mental alertness and motor coordination, such as driving a motor vehicle or operating machinery, until the next day following a restful sleep. Patients will need to arrange transportation home following treatment with SPRAVATO.
5.9 Ulcerative or Interstitial Cystitis
Cases of ulcerative or interstitial cystitis have been reported in individuals with long-term off-label use or misuse/abuse of ketamine. In clinical studies with SPRAVATO nasal spray, there was a higher rate of lower urinary tract symptoms (pollakiuria, dysuria, micturition urgency, nocturia, and cystitis) in SPRAVATO-treated patients than in placebo-treated patients [see Adverse Reactions (6)] . No cases of esketamine-related interstitial cystitis were observed in any of the studies, which included treatment for up to a year.
Monitor for urinary tract and bladder symptoms during the course of treatment with SPRAVATO, and refer to an appropriate healthcare provider as clinically warranted.
5.10 Embryo-fetal Toxicity
Based on published findings from pregnant animals treated with ketamine, the racemic mixture of arketamine and esketamine, SPRAVATO may cause fetal harm when administered to pregnant women. Advise pregnant women of the potential risk to an infant exposed to SPRAVATO in utero. Advise women of reproductive potential to consider pregnancy planning and prevention [see Use in Specific Populations (8.1, 8.3)].
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Sedation [see Warnings and Precautions (5.1)]
- Dissociation [see Warnings and Precautions (5.2)]
- Increase in Blood Pressure [see Warnings and Precautions (5.6)]
- Cognitive Impairment [see Warnings and Precautions (5.7)]
- Impaired Ability to Drive and Operate Machinery [see Warnings and Precautions (5.8)]
- Ulcerative or Interstitial Cystitis [see Warnings and Precautions (5.9)]
- Embryo-fetal Toxicity [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Dissociation/Perceptual Changes
SPRAVATO can cause dissociative symptoms (including derealization and depersonalization) and perceptual changes (including distortion of time and space, and illusions). In clinical trials, dissociation was transient and occurred on the day of dosing. Dissociation was evaluated by adverse event reports and the Clinician-Administered Dissociative States Scale (CADSS). A CADSS total score of more than 4 indicates the presence of dissociative symptoms, and such an increase to a score of 4 or more occurred in a higher number of patients on SPRAVATO compared to placebo during the short-term TRD studies. Dose-related increases in the incidence of dissociative symptoms (CADSS total score >4 and change >0) were observed in a fixed-dose TRD study [see Warnings and Precautions (5.2)] . Table 6 presents the incidence of dissociation (CADSS total score >4 and change >0) in a fixed-dose study with adult patients <65 years of age with TRD and a flexible-dose study with patients ≥65 years of age with TRD.
| Patients <65 years | Patients ≥65 years | ||||
|---|---|---|---|---|---|
| Placebo + Oral AD | SPRAVATO + Oral AD | Placebo + Oral AD | SPRAVATO + Oral AD | ||
| 56 mg | 84 mg | 28 to 84 mg | |||
|
|||||
| Number of patients* | N=113 | N=113 | N=116 | N=65 | N=72 |
| CADSS total score >4 and change >0 | 5% | 61% | 69% | 12% | 75% |
In studies for the treatment of depressive symptoms in adults with MDD with acute suicidal ideation or behavior, patients treated with SPRAVATO plus oral AD also demonstrated a higher number (84%) with dissociation (CADSS total score >4 and change >0) compared to patients treated with placebo plus oral AD (16%).
7. Drug Interactions
7.1 Central Nervous System Depressants
Concomitant use with CNS depressants (e.g., benzodiazepines, opioids, alcohol) may increase sedation [see Warnings and Precautions (5.1)] . Closely monitor for sedation with concomitant use of SPRAVATO with CNS depressants.
8. Use In Specific Populations
8.2 Lactation
Risk Summary
Esketamine is present in human milk. There are no data on the effects of SPRAVATO on the breastfed infant or on milk production. Published studies in juvenile animals report neurotoxicity (see Data). Because of the potential for neurotoxicity, advise patients that breast-feeding is not recommended during treatment with SPRAVATO.
Data
Published juvenile animal studies demonstrate that the administration of drugs that block NMDA receptors, such as ketamine, during the period of rapid brain growth or synaptogenesis, results in widespread neuronal and oligodendrocyte cell loss in the developing brain and alterations in synaptic morphology and neurogenesis. Based on comparisons across species, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester of gestation through the first several months of life, but this window may extend out to approximately 3 years of age in humans.
8.4 Pediatric Use
The safety and effectiveness of SPRAVATO in pediatric patients have not been established. Clinical studies of SPRAVATO in pediatric patients have not been conducted.
8.5 Geriatric Use
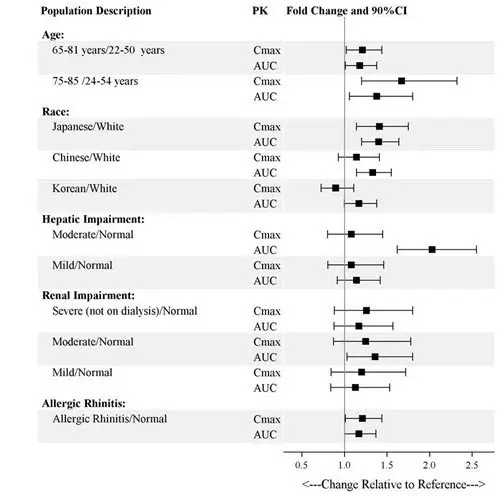
Of the total number of patients in Phase 3 clinical studies exposed to SPRAVATO, (N=1601), 194 (12%) were 65 years of age and older, and 25 (2%) were 75 years of age and older. No overall differences in the safety profile were observed between patients 65 years of age and older and patients younger than 65 years of age.
The mean esketamine C max and AUC values were higher in elderly patients compared with younger adult patients [see Clinical Pharmacology (12.3)] .
The efficacy of SPRAVATO for the treatment of TRD in geriatric patients was evaluated in a 4-week, randomized, double-blind study comparing flexibly-dosed intranasal SPRAVATO plus a newly initiated oral antidepressant compared to intranasal placebo plus a newly initiated oral antidepressant in patients ≥ 65 years of age. SPRAVATO was initiated at 28 mg twice weekly and could be titrated to 56 mg or 84 mg administered twice-weekly. At the end of four weeks, there was no statistically significant difference between groups on the primary efficacy endpoint of change from baseline to Week 4 on the Montgomery-Åsberg Depression Rating Scale (MADRS).
8.6 Hepatic Impairment
The mean esketamine AUC and t 1/2 values were higher in patients with moderate hepatic impairment compared to those with normal hepatic function [see Clinical Pharmacology (12.3)] . SPRAVATO-treated patients with moderate hepatic impairment may need to be monitored for adverse reactions for a longer period of time.
SPRAVATO has not been studied in patients with severe hepatic impairment (Child-Pugh class C). Use in this population is not recommended [see Clinical Pharmacology (12.3)].
9. Drug Abuse and Dependence
9.1 Controlled Substance
SPRAVATO contains esketamine hydrochloride, the (S)-enantiomer of ketamine and a Schedule III controlled substance under the Controlled Substances Act.
9.2 Abuse
Individuals with a history of drug abuse or dependence may be at greater risk for abuse and misuse of SPRAVATO. Abuse is the intentional, non-therapeutic use of a drug, even once, for its psychological or physiological effects. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a healthcare provider or for whom it was not prescribed. Careful consideration is advised prior to use of individuals with a history of substance use disorder, including alcohol.
SPRAVATO may produce a variety of symptoms including anxiety, dysphoria, disorientation, insomnia, flashback, hallucinations, and feelings of floating, detachment and to be "spaced out". Monitoring for signs of abuse and misuse is recommended.
9.3 Dependence
Physical dependence has been reported with prolonged use of ketamine. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or significant dosage reduction of a drug. There were no withdrawal symptoms captured up to 4 weeks after cessation of esketamine treatment. Withdrawal symptoms have been reported after the discontinuation of frequently used (more than weekly) large doses of ketamine for long periods of time. Such withdrawal symptoms are likely to occur if esketamine were similarly abused. Reported symptoms of withdrawal associated with daily intake of large doses of ketamine include craving, fatigue, poor appetite, and anxiety. Therefore, monitor SPRAVATO-treated patients for symptoms and signs of physical dependence upon the discontinuation of the drug.
Tolerance has been reported with prolonged use of ketamine. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose). Similar tolerance would be expected with prolonged use of esketamine.
11. Spravato Description
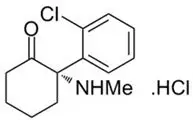
SPRAVATO ® contains esketamine hydrochloride, a non-competitive N-methyl- D-aspartate (NMDA) receptor antagonist. Esketamine is the S-enantiomer of racemic ketamine. The chemical name is (S)-2-(o-chlorophenyl)-2-(methylamino)cyclohexanone hydrochloride. Its molecular formula is C 13H 16ClNO.HCl and its molecular weight is 274.2. The structural formula is:
Esketamine hydrochloride is a white or almost white crystalline powder that is freely soluble in water and in methanol, and soluble in ethanol.
SPRAVATO nasal spray is intended for nasal administration. Esketamine hydrochloride is contained as a solution in a stoppered glass vial within the nasal spray device. Each device delivers two sprays with a total of 32.3 mg of esketamine hydrochloride (equivalent to 28 mg of esketamine) in 0.2 mL of a clear, colorless aqueous solution with a pH of 4.5.
The inactive ingredients are citric acid monohydrate, edetate disodium, sodium hydroxide, and water for injection.
12. Spravato - Clinical Pharmacology
12.1 Mechanism of Action
Esketamine, the S-enantiomer of racemic ketamine, is a non-selective, non-competitive antagonist of the N-methyl- D-aspartate (NMDA) receptor, an ionotropic glutamate receptor. The mechanism by which esketamine exerts its antidepressant effect is unknown. The major circulating metabolite of esketamine (noresketamine) demonstrated activity at the same receptor with less affinity.
12.3 Pharmacokinetics
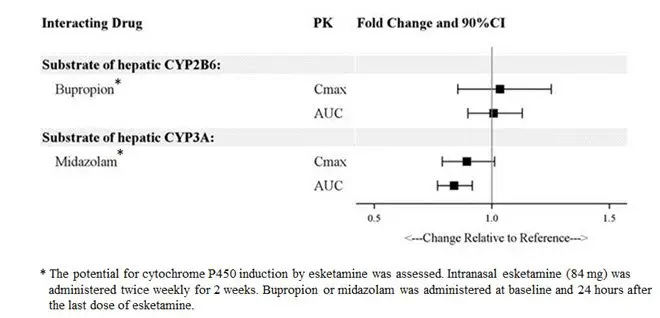
Esketamine exposure increases with dose from 28 mg to 84 mg. The increase in C max and AUC values was less than dose-proportional between 28 mg and 56 mg or 84 mg, but it was nearly dose proportional between 56 mg and 84 mg. No accumulation of esketamine in plasma was observed following twice a week administration.
13. Nonclinical Toxicology
14. Clinical Studies
14.1 Treatment-Resistant Depression
Short-Term Study
SPRAVATO was evaluated in a randomized, placebo-controlled, double-blind, multicenter, short-term (4-week), Phase 3 study (Study 1; NCT02418585) in adult patients 18 to <65 years old with treatment-resistant depression (TRD). Patients in Study 1 met DSM-5 criteria for major depressive disorder (MDD) and in the current depressive episode, had not responded adequately to at least two different antidepressants of adequate dose and duration. After discontinuing prior antidepressant treatments, patients in Study 1 were randomized to receive twice weekly doses of intranasal SPRAVATO (flexible dose; 56 mg or 84 mg) or intranasal placebo. All patients also received open-label concomitant treatment with a newly initiated daily oral antidepressant (AD) (duloxetine, escitalopram, sertraline, or extended-release venlafaxine as determined by the investigator based on patient's prior treatment history). SPRAVATO could be titrated up to 84 mg starting with the second dose based on investigator discretion.
The demographic and baseline disease characteristics of patients in Study 1 were similar for the SPRAVATO and placebo nasal spray groups. Patients had a median age of 47 years (range 19 to 64 years) and were 62% female, 93% Caucasian, and 5% Black. The newly initiated oral AD was an SSRI in 32% of patients and an SNRI in 68% of patients.
In Study 1, the primary efficacy measure was change from baseline in the Montgomery-Åsberg Depression Rating Scale (MADRS) total score at the end of the 4-week double-blind induction phase. The MADRS is a ten-item, clinician-rated scale used to assess severity of depressive symptoms. Scores on the MADRS range from 0 to 60, with higher scores indicating more severe depression. SPRAVATO plus a newly initiated oral AD demonstrated statistical superiority on the primary efficacy measure compared to placebo nasal spray plus a newly initiated oral AD (see Table 9).
| Treatment Group | Number of Patients | Mean Baseline Score (SD) | LS Mean (SE) Change from Baseline to end of Week 4 | LS Mean Difference
(95% CI) * |
|---|---|---|---|---|
| SD=standard deviation; SE=standard error; LS Mean=least-squares mean; CI=confidence interval; AD=antidepressant | ||||
|
||||
| SPRAVATO (56 mg or 84 mg) + Oral AD † | 114 | 37.0 (5.7) | -19.8 (1.3) | -4.0
(-7.3; -0.6) |
| Placebo nasal spray + Oral AD | 109 | 37.3 (5.7) | -15.8 (1.3) | - |
Treatment-Resistant Depression – Long-term Study
Study 2 (NCT02493868) was a long-term randomized, double-blind, parallel-group, multicenter maintenance-of-effect study in adults 18 to <65 years of age who were known remitters and responders to SPRAVATO. Patients in this study were responders in one of two short-term controlled trials (Study 1 and another 4-week study) or in an open-label direct-enrollment study in which they received flexibly-dosed SPRAVATO (56 mg or 84 mg twice weekly) plus daily oral AD in an initial 4-week phase.
Stable remission was defined as a MADRS total score ≤ 12 for at least 3 of the last 4 weeks. Stable response was defined as a MADRS total score reduction ≥ 50% for the last 2 weeks of optimization and not in remission. After at least 16 initial weeks of treatment with SPRAVATO and an oral AD, stable remitters and stable responders were randomized separately to continue intranasal treatment with SPRAVATO or switch to placebo nasal spray, in both cases with continuation of their oral AD. The primary study endpoint was time to relapse in the stable remitter group. Relapse was defined as a MADRS total score ≥22 for 2 consecutive weeks or hospitalization for worsening depression or any other clinically relevant event indicative of relapse.
The demographic and baseline disease characteristics of the two groups were similar. Patients had a median age of 48 years (range 19 to 64 years) and were 66% female, 90% Caucasian, and 4% Black.
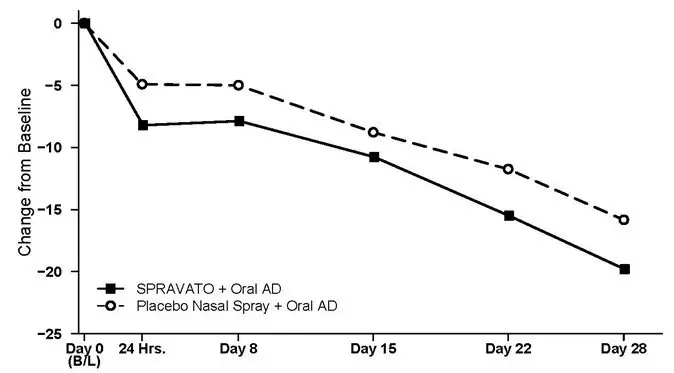
Patients in stable remission who continued treatment with SPRAVATO plus oral AD experienced a statistically significantly longer time to relapse of depressive symptoms than did patients on placebo nasal spray plus an oral AD (see Figure 5).
|
| Figure 5: Time to Relapse in Patients with TRD in Stable Remission in Study 2 * (Full Analysis Set) |
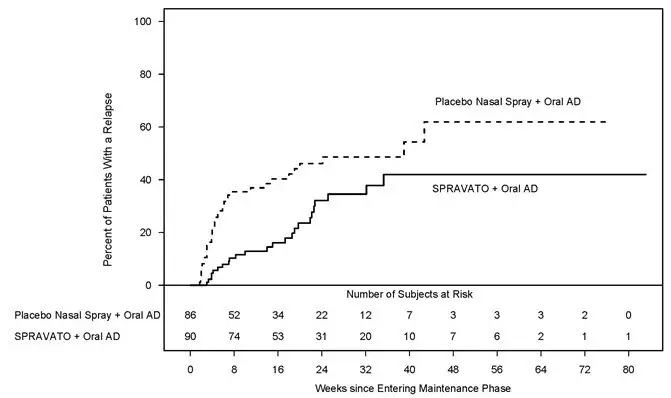 |
Time to relapse was also significantly delayed in the stable responder population. These patients experienced a statistically significantly longer time to relapse of depressive symptoms than patients on placebo nasal spray plus oral AD (see Figure 6).
|
| Figure 6: Time to Relapse in Patients in Stable Response in Patients with TRD in Study 2 * (Full Analysis Set) |
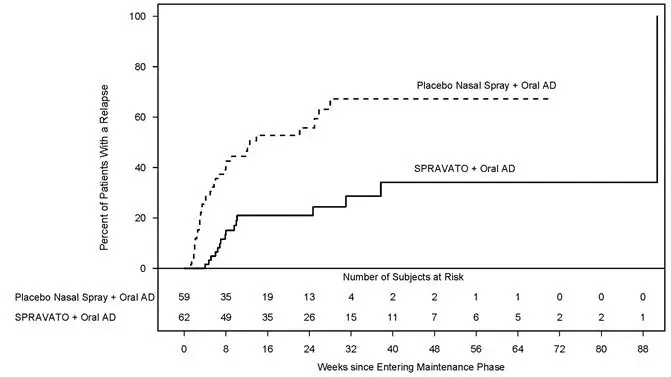 |
In Study 2, based on depressive symptomatology, the majority of stable remitters (69%) received every-other-week dosing for the majority of time during the maintenance phase; 23% of stable remitters received weekly dosing. Among stable responders, 34% received every-other-week dosing and 55% received weekly dosing the majority of time during the maintenance phase. Of the patients randomized to SPRAVATO, 39% received the 56 mg dose and 61% received the 84 mg dose.
14.2 Depressive Symptoms in Patients with Major Depressive Disorder with Acute Suicidal Ideation or Behavior
SPRAVATO was evaluated in two identical Phase 3 short-term (4-week) randomized, double-blind, multicenter, placebo-controlled studies, Study 3 (NCT03039192) and Study 4 (NCT03097133), in adults with moderate-to-severe MDD (MADRS total score >28) who had active suicidal ideation and intent. In these studies, patients received treatment with SPRAVATO 84 mg or placebo nasal spray twice-weekly for 4 weeks. After the first dose, a one-time dose reduction to SPRAVATO 56 mg was allowed for patients unable to tolerate the 84 mg dose. All patients received comprehensive standard of care treatment, including an initial inpatient psychiatric hospitalization and a newly initiated or optimized oral antidepressant (AD) (AD monotherapy or AD plus augmentation therapy) as determined by the investigator. After completion of the 4-week treatment period with SPRAVATO/placebo, study follow-up continued through Day 90.
The baseline demographic and disease characteristics of patients in Study 3 and Study 4 were similar between the SPRAVATO plus standard of care or placebo nasal spray plus standard of care treatment groups. The median patient age was 40 years (range 18 to 64 years), 61% were female; 73% Caucasian and 6% Black; and 63% of patients had at least one prior suicide attempt. Prior to entering the study, 92% of the patients were receiving antidepressant therapy. During the study, as part of standard of care treatment, 40% of patients received AD monotherapy, 54% of patients received AD plus augmentation therapy, and 6% received both AD monotherapy/AD plus augmentation therapy.
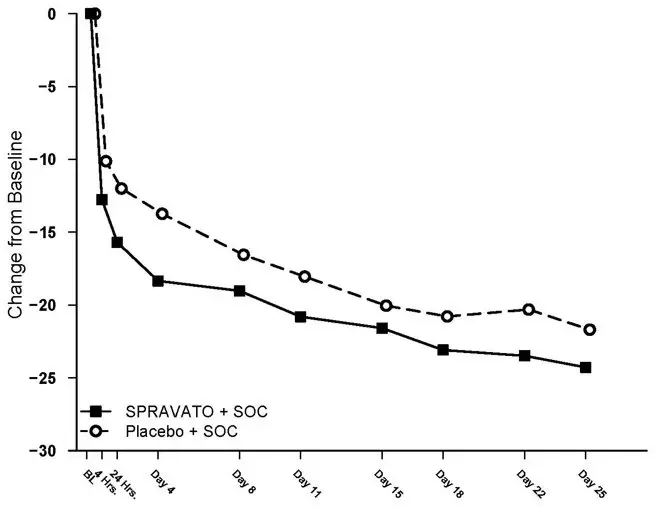
The primary efficacy measure was the change from baseline in the MADRS total score at 24 hours after first dose (Day 2). In Study 3 and Study 4, SPRAVATO plus standard of care demonstrated statistical superiority on the primary efficacy measure compared to placebo nasal spray plus standard of care (see Table 10).
| Study No. | Treatment Group * | Number of Patients | Mean Baseline Score (SD) | LS Mean Change from Baseline to 24 hr Post First Dose (SE) | LS Mean Difference (95% CI) † |
|---|---|---|---|---|---|
| SD=standard deviation; SE=standard error; LS Mean=least-squares mean; CI=confidence interval; SOC=standard of care. | |||||
|
|||||
| Study 3 | SPRAVATO 84 mg + SOC ‡ | 111 | 41.3 (5.87) | -15.9 (1.04) | -3.8
(-6.56; -1.09) |
| Placebo nasal spray + SOC | 112 | 41.0 (6.29) | -12.0 (1.02) | - | |
| Study 4 | SPRAVATO 84 mg + SOC ‡ | 113 | 39.4 (5.21) | -16.0 (1.02) | -3.9
(-6.60; -1.11) |
| Placebo nasal spray + SOC | 113 | 39.9 (5.76) | -12.2 (1.05) | - | |
The secondary efficacy measure was the change in Clinical Global Impression of Suicidal Severity - Revised (CGI-SS-r) score at 24 hours after first dose (Day 2). The CGI-SS-r is a one-item, clinician-rated assessment used to rate the current severity of a patient's suicidal ideation and behavior. Scores on the CGI-SS-r range from 0 to 6, with higher scores indicating more severe suicidal ideation and behavior. In Study 3 and Study 4, SPRAVATO plus standard of care did not demonstrate superiority compared to placebo nasal spray plus standard of care in improving CGI-SS-r.
16. How is Spravato supplied
SPRAVATO ® nasal spray is available as an aqueous solution of esketamine hydrochloride in a stoppered glass vial within a nasal spray device. Each nasal spray device delivers two sprays containing a total of 28 mg of esketamine (supplied as 32.3 mg of esketamine hydrochloride).
SPRAVATO is available in the following presentations:
- 56 mg Dose Kit: Unit-dose carton containing two 28 mg nasal spray devices (56 mg total dose) (NDC 50458-028-02).
- 84 mg Dose Kit: Unit-dose carton containing three 28 mg nasal spray devices (84 mg total dose) (NDC 50458-028-03).
Within each kit, each 28 mg device is individually packaged in a sealed blister (NDC 50458-028-00).
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: 07/2020 | |
|
MEDICATION GUIDE
|
||
|
What is the most important information I should know about SPRAVATO? SPRAVATO can cause serious side effects, including:
|
||
|
|
|
|
What is SPRAVATO? SPRAVATO is a prescription medicine used along with an antidepressant taken by mouth to treat:
SPRAVATO is not for use as a medicine to prevent or relieve pain (anesthetic). It is not known if SPRAVATO is safe and effective as an anesthetic medicine. It is not known if SPRAVATO is safe and effective for use in preventing suicide or in reducing suicidal thoughts or actions. SPRAVATO is not for use in place of hospitalization if your healthcare provider determines that hospitalization is needed, even if improvement is experienced after the first dose of SPRAVATO. It is not known if SPRAVATO is safe and effective in children. |
||
|
Do not take SPRAVATO if you:
If you are not sure if you have any of the above conditions, talk to your healthcare provider before taking SPRAVATO. |
||
|
Before you take SPRAVATO, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines that you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Taking SPRAVATO with certain medicines may cause side effects.
Especially tell your healthcare provider if you take central nervous system (CNS) depressants, psychostimulants, or monoamine oxidase inhibitors (MAOIs) medicines. Ask your healthcare provider if you are not sure if you take these medicines. Your healthcare provider can tell you if it is safe to take SPRAVATO with your other medicines.
|
||
|
How will I take SPRAVATO?
|
||
|
What should I avoid while taking SPRAVATO?
|
||
|
What are the possible side effects of SPRAVATO? SPRAVATO may cause serious side effects, including:
The most common side effects of SPRAVATO when used along with an antidepressant taken by mouth include: |
||
|
|
|
|
If these common side effects occur, they usually happen right after taking SPRAVATO and go away the same day. These are not all the possible side effects of SPRAVATO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
|
General information about SPRAVATO. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about SPRAVATO that is written for healthcare providers. |
||
|
What are the ingredients in SPRAVATO?
Active ingredient: esketamine hydrochloride
Manufactured for: Janssen Pharmaceuticals, Inc., Titusville, NJ 08560
|
||
| SPRAVATO
esketamine hydrochloride solution |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Janssen Pharmaceuticals Inc. (063137772) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Pharmaceutica NV | 400345889 | api manufacture(50458-028) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Pharmaceuticals, Inc. | 080236951 | api manufacture(50458-028) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Renaissance Lakewood LLC | 077744035 | analysis(50458-028) , manufacture(50458-028) , pack(50458-028) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CU Chemie Uetikon GmbH | 340717289 | api manufacture(50458-028) , analysis(50458-028) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PPD Development, LP. | 838082055 | analysis(50458-028) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen-Cilag Manufacturing, LLC | 963971374 | analysis(50458-028) , pack(50458-028) , manufacture(50458-028) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Ortho LLC | 805887986 | pack(50458-028) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Pharmaceuticals, Inc | 868441320 | analysis(50458-028) | |