Drug Detail:Striverdi respimat (Olodaterol (inhalation) [ oh-loe-dat-er-ol ])
Drug Class: Adrenergic bronchodilators
Highlights of Prescribing Information
STRIVERDI® RESPIMAT® (olodaterol inhalation spray), for oral inhalation use
Initial U.S. Approval: 2014
Indications and Usage for Striverdi Respimat
STRIVERDI RESPIMAT Inhalation Spray is a long-acting beta2-adrenergic agonist (LABA) indicated for:
The long-term, once-daily maintenance bronchodilator treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema (1.1)
Important limitations:
- STRIVERDI RESPIMAT is NOT indicated to treat acute deterioration of COPD (1.2)
- STRIVERDI RESPIMAT is NOT indicated to treat asthma (1.2)
Striverdi Respimat Dosage and Administration
- For oral inhalation only
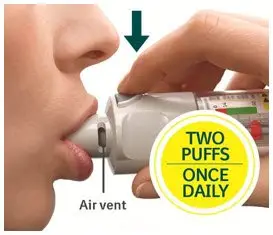
- Two inhalations of STRIVERDI RESPIMAT once-daily at the same time of day (2)
Dosage Forms and Strengths
Inhalation spray: Each actuation from the mouthpiece delivers 2.5 mcg olodaterol, equivalent to 2.7 mcg olodaterol hydrochloride. Two actuations equal one dose. (3)
Contraindications
Use of a LABA, including STRIVERDI RESPIMAT, without an inhaled corticosteroid is contraindicated in patients with asthma. (4)
Warnings and Precautions
- LABA as monotherapy (without an inhaled corticosteroid) for asthma increases the risk of serious asthma-related events. (5.1)
- Do not initiate STRIVERDI RESPIMAT in acutely deteriorating COPD patients (5.2)
- Do not use for relief of acute symptoms. Concomitant short-acting beta2-agonists can be used as needed for acute relief. (5.2)
- Do not exceed the recommended dose. Excessive use of STRIVERDI RESPIMAT, or use in conjunction with other medications containing LABA can result in clinically significant cardiovascular effects and may be fatal. (5.3)
- Life-threatening paradoxical bronchospasm can occur. Discontinue STRIVERDI RESPIMAT immediately. (5.4)
- Use with caution in patients with cardiovascular or convulsive disorders, thyrotoxicosis or sensitivity to sympathomimetic drugs (5.5, 5.6)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥2% and more than placebo) are nasopharyngitis, upper respiratory tract infection, bronchitis, urinary tract infection, cough, dizziness, rash, diarrhea, back pain and arthralgia (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Boehringer Ingelheim Pharmaceuticals, Inc. at (800) 542-6257 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Other adrenergic drugs may potentiate effect. Use with caution. (5.3, 7.1)
- Xanthine derivatives, steroids, diuretics or non-potassium sparing diuretics may potentiate hypokalemia or ECG changes. Use with caution. (7.2, 7.3)
- MAO inhibitors, tricyclic antidepressants, and drugs that prolong QTc interval may potentiate effect on cardiovascular system. Use with extreme caution. (7.4)
- Beta-blockers may decrease effectiveness. Use with caution and only when medically necessary. (7.5)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2021
Related/similar drugs
prednisone, Symbicort, Breo Ellipta, Ventolin, Spiriva, Ventolin HFA, Anoro ElliptaFull Prescribing Information
1. Indications and Usage for Striverdi Respimat
2. Striverdi Respimat Dosage and Administration
The recommended dose of STRIVERDI RESPIMAT is two inhalations once-daily at the same time of the day. Do not use STRIVERDI RESPIMAT more than two inhalations every 24 hours.
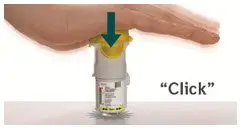
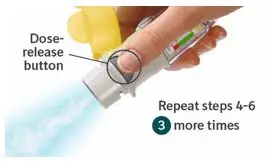
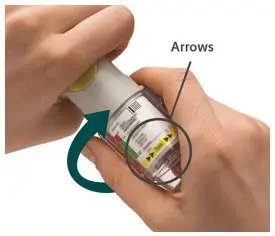
Prior to first use, the STRIVERDI RESPIMAT cartridge is inserted into the STRIVERDI RESPIMAT inhaler and the unit is primed. When using the unit for the first time, patients are to actuate the inhaler toward the ground until an aerosol cloud is visible and then repeat the process three more times. The unit is then considered primed and ready for use. If not used for more than 3 days, patients are to actuate the inhaler once to prepare the inhaler for use. If not used for more than 21 days, patients are to actuate the inhaler until an aerosol cloud is visible and then repeat the process three more times to prepare the inhaler for use [see Patient Counseling Information (17)].
No dosage adjustment is required for geriatric patients, patients with mild and moderate hepatic impairment, or renally-impaired patients. There are no data available for use of STRIVERDI RESPIMAT in severe hepatically impaired patients [see Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
STRIVERDI RESPIMAT consists of a STRIVERDI RESPIMAT inhaler and an aluminum cylinder (STRIVERDI RESPIMAT cartridge) containing olodaterol (as the hydrochloride). The STRIVERDI RESPIMAT cartridge is intended for use with the STRIVERDI RESPIMAT inhaler only.
Each actuation from the STRIVERDI RESPIMAT inhaler delivers 2.5 mcg olodaterol, equivalent to 2.7 mcg olodaterol hydrochloride, from the mouthpiece. Two actuations equal one dose.
4. Contraindications
Use of a LABA, including STRIVERDI RESPIMAT, without an inhaled corticosteroid is contraindicated in patients with asthma [see Warnings and Precautions (5.1)]. STRIVERDI RESPIMAT is not indicated for the treatment of asthma.
5. Warnings and Precautions
5.1 Serious Asthma-Related Events – Hospitalizations, Intubations, Death
- The safety and efficacy of STRIVERDI RESPIMAT in patients with asthma have not been established. STRIVERDI RESPIMAT is not indicated for the treatment of asthma [see Contraindications (4)].
- Use of long-acting beta2-adrenergic agonists (LABA) as monotherapy [without inhaled corticosteroids (ICS)] for asthma is associated with an increased risk of asthma-related death. Available data from controlled clinical trials also suggest that use of LABA as monotherapy increases the risk of asthma-related hospitalization in pediatric and adolescent patients. These findings are considered a class effect of LABA monotherapy. When LABA are used in fixed-dose combination with ICS, data from large clinical trials do not show a significant increase in the risk of serious asthma-related events (hospitalizations, intubations, death) compared with ICS alone.
- A 28-week, placebo-controlled US study comparing the safety of another LABA (salmeterol) with placebo, each added to usual asthma therapy, showed an increase in asthma-related deaths in patients receiving salmeterol (13/13,176 in patients treated with salmeterol vs. 3/13,179 in patients treated with placebo; RR 4.37, 95% CI 1.25, 15.34). The increased risk of asthma-related death is considered a class effect of LABA, including STRIVERDI RESPIMAT.
- No study adequate to determine whether the rate of asthma-related death is increased in patients treated with STRIVERDI RESPIMAT has been conducted.
- Available data do not suggest an increased risk of death with use of LABA in patients with COPD.
5.2 Deterioration of Disease and Acute Episodes
STRIVERDI RESPIMAT should not be initiated in patients with acutely deteriorating COPD, which may be a life-threatening condition. STRIVERDI RESPIMAT has not been studied in patients with acutely deteriorating COPD. The use of STRIVERDI RESPIMAT in this setting is inappropriate.
STRIVERDI RESPIMAT should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. STRIVERDI RESPIMAT has not been studied in the relief of acute symptoms and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled short-acting beta2-agonist.
When beginning STRIVERDI RESPIMAT, patients who have been taking inhaled, short-acting beta2-agonists on a regular basis (e.g., four times a day) should be instructed to discontinue the regular use of these drugs and use them only for symptomatic relief of acute respiratory symptoms. When prescribing STRIVERDI RESPIMAT, the healthcare provider should also prescribe an inhaled, short-acting beta2-agonist and instruct the patient on how it should be used. Increasing inhaled beta2-agonist use is a signal of deteriorating disease for which prompt medical attention is indicated.
COPD may deteriorate acutely over a period of hours or chronically over several days or longer. If STRIVERDI RESPIMAT no longer controls symptoms of bronchoconstriction, or the patient's inhaled, short-acting beta2-agonist becomes less effective or the patient needs more inhalation of short-acting beta2-agonist than usual, these may be markers of deterioration of disease. In this setting, a re-evaluation of the patient and the COPD treatment regimen should be undertaken at once. Increasing the daily dosage of STRIVERDI RESPIMAT beyond the recommended dose is not appropriate in this situation.
5.3 Excessive Use of STRIVERDI RESPIMAT and Use with Long-Acting Beta2-Agonists
As with other inhaled drugs containing beta2-adrenergic agents, STRIVERDI RESPIMAT should not be used more often than recommended, at higher doses than recommended, or in conjunction with other medications containing long-acting beta2-agonists, as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs.
5.4 Paradoxical Bronchospasm
As with other inhaled beta2-agonists, STRIVERDI RESPIMAT may produce paradoxical bronchospasm that may be life-threatening. If paradoxical bronchospasm occurs, STRIVERDI RESPIMAT should be discontinued immediately and alternative therapy instituted.
5.5 Cardiovascular Effects
STRIVERDI RESPIMAT, like other beta2-agonists, can produce a clinically significant cardiovascular effect in some patients as measured by increases in pulse rate, systolic or diastolic blood pressure, and/or symptoms. If such effects occur, STRIVERDI RESPIMAT may need to be discontinued. In addition, beta-agonists have been reported to produce ECG changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Long acting beta2-adrenergic agonists should be administered with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, hypertrophic obstructive cardiomyopathy, and hypertension.
5.6 Co-existing Conditions
STRIVERDI RESPIMAT, like other sympathomimetic amines, should be used with caution in patients with convulsive disorders or thyrotoxicosis, in patients with known or suspected prolongation of the QT interval, and in patients who are unusually responsive to sympathomimetic amines. Doses of the related beta2-agonist albuterol, when administered intravenously, have been reported to aggravate pre-existing diabetes mellitus and ketoacidosis.
5.7 Hypokalemia and Hyperglycemia
Beta-adrenergic agonists may produce significant hypokalemia in some patients, which has the potential to produce adverse cardiovascular effects [see Clinical Pharmacology (12.2)]. The decrease in serum potassium is usually transient, not requiring supplementation. Inhalation of high doses of beta2-adrenergic agonists may produce increases in plasma glucose.
In patients with severe COPD, hypokalemia may be potentiated by hypoxia and concomitant treatment [see Drug Interactions (7.2)], which may increase the susceptibility for cardiac arrhythmias.
Clinically notable decreases in serum potassium or changes in blood glucose were infrequent during clinical studies with long-term administration of STRIVERDI RESPIMAT with the rates similar to those for placebo controls. STRIVERDI RESPIMAT has not been investigated in patients whose diabetes mellitus is not well controlled.
6. Adverse Reactions/Side Effects
Long-acting beta2-adrenergic agonists, such as STRIVERDI RESPIMAT, as monotherapy (without an inhaled corticosteroid) for asthma, increase the risk of asthma-related events. STRIVERDI RESPIMAT is not indicated for the treatment of asthma [see Warnings and Precautions (5.1)].
6.1 Clinical Trials Experience in Chronic Obstructive Pulmonary Disease
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The STRIVERDI RESPIMAT clinical development program included seven dose-ranging trials and eight confirmatory trials. Four of the confirmatory trials were 6-week cross-over trials and four were 48-week parallel group trials. Adverse reactions observed in the dose-ranging trials and four 6-week cross-over trials were consistent with those observed in the 48-week parallel group trials, which formed the primary safety database.
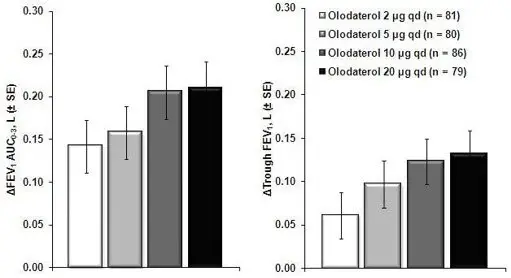
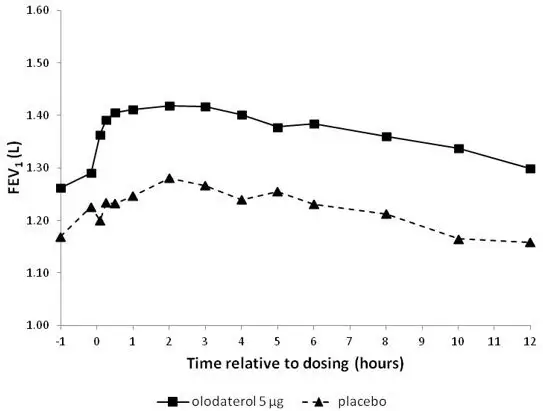
The primary safety database consisted of pooled data from the four 48-week double-blind, active and placebo-controlled, parallel group confirmatory clinical trials. These trials included 3104 adult COPD patients (77% males and 23% females) 40 years of age and older. Of these patients, 876 and 883 patients were treated with STRIVERDI RESPIMAT 5 mcg and 10 mcg once-daily, respectively. The STRIVERDI RESPIMAT groups were composed of mostly Caucasians (66%) with a mean age of 64 years and a mean percent predicted FEV1 at baseline of 44% for both the 5 mcg and 10 mcg treatment groups. Control arms for comparison included placebo in all four trials plus formoterol 12 mcg in two trials.
In these four clinical trials, seventy-two percent (72%) of patients exposed to any dose of STRIVERDI RESPIMAT reported an adverse reaction compared to 71% in the placebo group. The proportion of patients who discontinued due to an adverse reaction was 7.2% for STRIVERDI RESPIMAT treated patients compared to 8.8% for placebo treated patients. The adverse reaction most commonly leading to discontinuation was worsening COPD. The most common serious adverse reactions were COPD exacerbation, pneumonia, and atrial fibrillation.
Table 1 shows all adverse drug reactions reported by at least 2% of patients (and higher than placebo) who received STRIVERDI RESPIMAT 5 mcg during the 48-week trials.
| Treatment | STRIVERDI 5 mcg once-daily | Placebo |
|---|---|---|
| Body system (adverse drug reaction) | n=876 n (%) | n=885 n (%) |
| *Rash includes a grouping of similar terms | ||
| Infections and infestations | ||
| Nasopharyngitis | 99 (11.3) | 68 (7.7) |
| Upper Respiratory Tract Infection | 72 (8.2) | 66 (7.5) |
| Bronchitis | 41 (4.7) | 32 (3.6) |
| Urinary Tract Infection | 22 (2.5) | 9 (1.0) |
| Respiratory, thoracic, and mediastinal disorders | ||
| Cough | 37 (4.2) | 35 (4.0) |
| Nervous system disorders | ||
| Dizziness | 20 (2.3) | 19 (2.1) |
| Skin and subcutaneous tissue disorders | ||
| Rash* | 19 (2.2) | 10 (1.1) |
| Gastrointestinal disorders | ||
| Diarrhea | 25 (2.9) | 22 (2.5) |
| Musculoskeletal and connective tissue disorders | ||
| Back Pain | 31 (3.5) | 24 (2.7) |
| Arthralgia | 18 (2.1) | 7 (0.8) |
Additional adverse reactions that occurred in greater than 2% (and higher than placebo) of patients exposed to STRIVERDI RESPIMAT 10 mcg were pneumonia, constipation, and pyrexia.
Lung cancers were reported in 6 (0.7%), 3 (0.3%), and 2 (0.2%) patients who received STRIVERDI RESPIMAT 10 mcg, 5 mcg, and placebo, respectively.
7. Drug Interactions
7.1 Adrenergic Drugs
If additional adrenergic drugs are to be administered by any route, they should be used with caution because the sympathetic effects of STRIVERDI RESPIMAT may be potentiated [see Warnings and Precautions (5.3, 5.5, 5.6, 5.7)].
7.2 Xanthine Derivatives, Steroids, or Diuretics
Concomitant treatment with xanthine derivatives, steroids, or diuretics may potentiate any hypokalemic effect of STRIVERDI RESPIMAT [see Warnings and Precautions (5.7)].
7.3 Non-Potassium Sparing Diuretics
The ECG changes and/or hypokalemia that may result from the administration of non-potassium sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta-agonists, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the co-administration of beta-agonists with non-potassium-sparing diuretics.
7.4 Monoamine Oxidase Inhibitors, Tricyclic Antidepressants, QTc Prolonging Drugs
STRIVERDI RESPIMAT, as with other beta2-agonists, should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants or other drugs known to prolong the QTc interval because the action of adrenergic agonists on the cardiovascular system may be potentiated by these agents. Drugs that are known to prolong the QTc interval may be associated with an increased risk of ventricular arrhythmias.
7.5 Beta-Blockers
Beta-adrenergic receptor antagonists (beta-blockers) and STRIVERDI RESPIMAT may interfere with the effect of each other when administered concurrently. Beta-blockers not only block the therapeutic effects of beta-agonists, but may produce severe bronchospasm in COPD patients. Therefore, patients with COPD should not normally be treated with beta-blockers. However, under certain circumstances, e.g. as prophylaxis after myocardial infarction, there may be no acceptable alternatives to the use of beta-blockers in patients with COPD. In this setting, cardioselective beta-blockers could be considered, although they should be administered with caution.
7.6 Inhibitors of Cytochrome P450 and P-gp Efflux Transporter
In a drug interaction study using the strong dual CYP and P-gp inhibitor ketoconazole, a 1.7-fold increase of maximum plasma concentrations and AUC was observed [see Clinical Pharmacology (12.3)]. STRIVERDI RESPIMAT was evaluated in clinical trials for up to one year at doses up to twice the recommended therapeutic dose. No dose adjustment is necessary.
8. Use In Specific Populations
8.4 Pediatric Use
STRIVERDI RESPIMAT is not indicated for use in children. The safety and effectiveness of STRIVERDI RESPIMAT in the pediatric population have not been established.
8.5 Geriatric Use
Based on available data, no adjustment of STRIVERDI RESPIMAT dosage in geriatric patients is necessary.
Of the 876 patients who received STRIVERDI RESPIMAT at the recommended dose of 5 mcg once-daily in the clinical studies from the pooled 1-year database, 485 were less than or equal to 65 years of age and 391 (44.6%) were greater than 65 years of age.
No overall differences in effectiveness were observed, and in the 1-year pooled data, the adverse drug reaction profiles were similar in the older population compared to the patient population overall.
10. Overdosage
The expected signs and symptoms with overdosage of STRIVERDI RESPIMAT are those of excessive beta-adrenergic stimulation and occurrence or exaggeration of any of the signs and symptoms, e.g., myocardial ischemia, angina pectoris, hypertension or hypotension, tachycardia, arrhythmias, palpitations, dizziness, nervousness, insomnia, anxiety, headache, tremor, dry mouth, muscle spasms, nausea, fatigue, malaise, hypokalemia, hyperglycemia, and metabolic acidosis. As with all inhaled sympathomimetic medications, cardiac arrest and even death may be associated with an overdose of STRIVERDI RESPIMAT.
Treatment of overdosage consists of discontinuation of STRIVERDI RESPIMAT together with institution of appropriate symptomatic and supportive therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. There is insufficient evidence to determine if dialysis is beneficial for overdosage of STRIVERDI RESPIMAT. Cardiac monitoring is recommended in cases of overdosage.
11. Striverdi Respimat Description
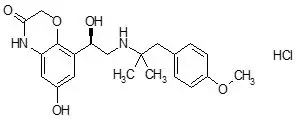
The active moiety olodaterol is a selective beta2-adrenergic bronchodilator. The drug substance, olodaterol hydrochloride, is chemically described as 2H-1,4-Benzoxazin-3H(4H)-one, 6-hydroxy-8-[(1R)-1-hydroxy-2-[[2-(4-methoxyphenyl)-1,1-dimethylethyl]-amino]ethyl]-, monohydrochloride. Olodaterol hydrochloride is a white to off-white powder that is sparingly-slightly soluble in water and slightly soluble in ethanol. The molecular weight is 422.9 g/mole (salt): 386.5 g/mole (base), and the molecular formula is C21H26N2O5 × HCl as a hydrochloride. The conversion factor from salt to free base is 1.094.
The structural formula is:
The drug product, STRIVERDI RESPIMAT, is composed of a sterile, aqueous solution of olodaterol hydrochloride filled into a 4.5 mL plastic container crimped into an aluminum cylinder (STRIVERDI RESPIMAT cartridge) for use with the STRIVERDI RESPIMAT inhaler.
Excipients include water for injection, benzalkonium chloride, edetate disodium, and anhydrous citric acid. The STRIVERDI RESPIMAT cartridge is only intended for use with the STRIVERDI RESPIMAT inhaler. The STRIVERDI RESPIMAT inhaler is a hand held, pocket sized oral inhalation device that uses mechanical energy to generate a slow-moving aerosol cloud of medication from a metered volume of the drug solution. The STRIVERDI RESPIMAT inhaler has a yellow-colored cap.
When used with the STRIVERDI RESPIMAT inhaler, each cartridge containing a minimum of 4 grams of a sterile aqueous solution delivers the labeled number of metered actuations after preparation for use. Each dose (1 dose equals 2 actuations) from the STRIVERDI RESPIMAT inhaler delivers 5 mcg olodaterol in 22.1 mcL of solution from the mouthpiece. As with all inhaled drugs, the actual amount of drug delivered to the lung may depend on patient factors, such as the coordination between the actuation of the inhaler and inspiration through the delivery system. The duration of inspiration should be at least as long as the spray duration (1.5 seconds).
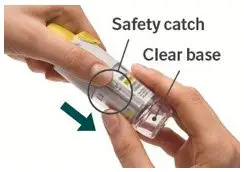
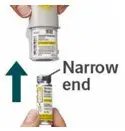
Prior to first use, the STRIVERDI RESPIMAT cartridge is inserted into the STRIVERDI RESPIMAT inhaler and the unit is primed. When using for the first time, patients are to actuate the inhaler toward the ground until an aerosol cloud is visible and then repeat the process three more times. The unit is then considered primed and ready for use. If not used for more than 3 days, patients are to actuate the inhaler once to prepare the inhaler for use. If not used for more than 21 days, patients are to actuate the inhaler until an aerosol cloud is visible and then repeat the process three more times to prepare the inhaler for use [see Patient Counseling Information (17)].
12. Striverdi Respimat - Clinical Pharmacology
12.1 Mechanism of Action
Olodaterol is a long-acting beta2-adrenergic agonist (LABA). The compound exerts its pharmacological effects by binding and activation of beta2-adrenoceptors after topical administration by inhalation. Activation of these receptors in the airways results in a stimulation of intracellular adenyl cyclase, an enzyme that mediates the synthesis of cyclic-3', 5' adenosine monophosphate (cAMP). Elevated levels of cAMP induce bronchodilation by relaxation of airway smooth muscle cells. In vitro studies have shown that olodaterol has 241-fold greater agonist activity at beta2-adrenoceptors compared to beta1-adrenoceptors and 2,299-fold greater agonist activity compared to beta3-adrenoceptors. The clinical significance of these findings is unknown.
Beta-adrenoceptors are divided into three subtypes: beta1-adrenoceptors predominantly expressed on cardiac smooth muscle, beta2-adrenoceptors predominantly expressed on airway smooth muscle, and beta3-adrenoceptors predominantly expressed on adipose tissue. Beta2-agonists cause bronchodilation. Although the beta2-adrenoceptor is the predominant adrenergic receptor in the airway smooth muscle, it is also present on the surface of a variety of other cells, including lung epithelial and endothelial cells and in the heart. The precise function of beta2-receptors in the heart is not known, but their presence raises the possibility that even highly selective beta2-agonists may have cardiac effects.
12.3 Pharmacokinetics
Olodaterol showed linear pharmacokinetics. On repeated once-daily inhalation steady-state of olodaterol plasma concentrations was achieved after 8 days, and the extent of exposure was increased up to 1.8-fold as compared to a single dose.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year inhalation studies were conducted in rats and mice to assess the carcinogenic potential of olodaterol. Lifetime treatment of female rats induced leiomyomas of the mesovarium at doses of 25.8 and 270 mcg/kg/day (approximately 18- and 198-fold, respectively, the MRHDID on an AUC basis). No tumor findings were observed in male rats at doses up to 270 mcg/kg/day (approximately 230-fold the MRHDID on an AUC basis). Lifetime treatment of female mice induced leiomyomas and leiomyosarcomas of the uterus at doses ≥76.9 mcg/kg/day (approximately 106-fold the MRHDID on an AUC basis). No tumor findings were observed in male mice at doses up to 255 mcg/kg/day (approximately 455-fold the MRHDID on an AUC basis). Increases in leiomyomas and leiomyosarcomas of the female rodent reproductive tract have been similarly demonstrated with other β2-adrenergic agonist drugs. The relevance of these findings to human use is unknown.
Olodaterol was not mutagenic in the in vitro Ames test or in the in vitro mouse lymphoma assay. Olodaterol produced increased frequency of micronuclei in rats after intravenous doses. The increased frequency of micronuclei was likely related to drug enhanced (compensatory) erythropoiesis. The mechanism for induction of micronuclei formation is likely not relevant at clinical exposures.
Olodaterol did not impair male or female fertility in rats at inhalation doses up to 3,068 mcg/kg/day (approximately 2,322 times the MRHDID on an AUC basis).
14. Clinical Studies
The STRIVERDI RESPIMAT clinical development program included three dose-ranging trials in COPD patients, four dose-ranging trials in asthma patients, and eight confirmatory trials in patients with COPD.
16. How is Striverdi Respimat supplied
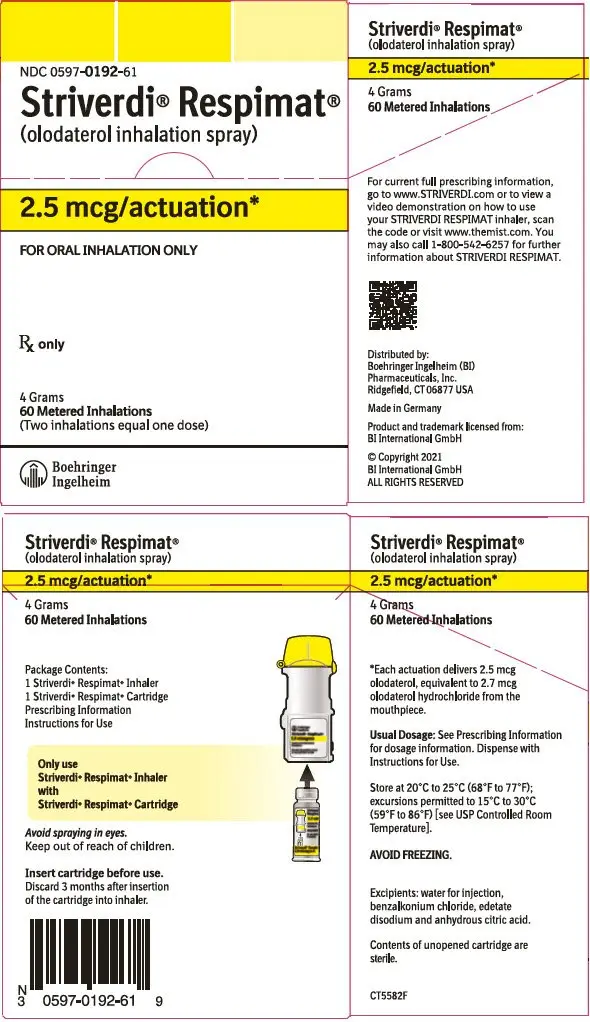
STRIVERDI RESPIMAT Inhalation Spray is supplied in a labeled carton containing one STRIVERDI RESPIMAT cartridge and one STRIVERDI RESPIMAT inhaler.
The STRIVERDI RESPIMAT cartridge is an aluminum cylinder with a tamper protection seal on the cap. The STRIVERDI RESPIMAT cartridge is only intended for use with the STRIVERDI RESPIMAT inhaler.
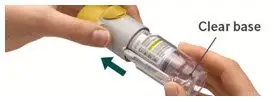
The STRIVERDI RESPIMAT inhaler is a cylindrical-shaped plastic inhalation device with a gray-colored body and a clear base. The clear base is removed to insert the cartridge. The inhaler contains a dose indicator. The yellow colored cap and the written information on the label of the gray inhaler body indicates that it is labeled for use with the STRIVERDI RESPIMAT cartridge.
STRIVERDI RESPIMAT Inhalation Spray is available as:
STRIVERDI RESPIMAT Inhalation Spray: 60 metered actuations (NDC 0597-0192-61)
The STRIVERDI RESPIMAT cartridge has a net fill weight of at least 4 grams and when used with the STRIVERDI RESPIMAT inhaler, is designed to deliver the labeled number of metered actuations after preparation for use.
When the labeled number of metered actuations has been dispensed from the inhaler, the STRIVERDI RESPIMAT locking mechanism will be engaged and no more actuations can be dispensed.
After assembly, the STRIVERDI RESPIMAT inhaler should be discarded at the latest 3 months after first use or when the locking mechanism is engaged, whichever comes first.
Keep out of reach of children. Do not spray into eyes.
| STRIVERDI RESPIMAT
olodaterol respimat inhalation spray spray, metered |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Registrant - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Pharma GmbH and Co. KG | 551147440 | API MANUFACTURE(0597-0192) , LABEL(0597-0192) , MANUFACTURE(0597-0192) , PACK(0597-0192) | |