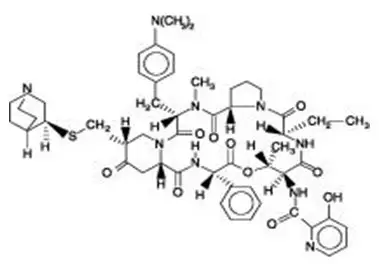
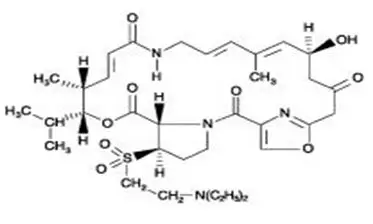
Drug Detail:Synercid (Dalfopristin and quinupristin [ dal-foe-pris-tin-and-kwi-nyoo-pris-tin ])
Drug Class: Streptogramins
Synercid - Clinical Pharmacology
Pharmacokinetics
Quinupristin and dalfopristin are the main active components circulating in plasma in human subjects. Quinupristin and dalfopristin are converted to several active major metabolites: two conjugated metabolites for quinupristin (one with glutathione and one with cysteine) and one non-conjugated metabolite for dalfopristin (formed by drug hydrolysis).
Pharmacokinetic profiles of quinupristin and dalfopristin in combination with their metabolites were determined using a bioassay following multiple 60-minute infusions of Synercid in two groups of healthy young adult male volunteers. Each group received 7.5 mg/kg of Synercid intravenously q12h or q8h for a total of 9 or 10 doses, respectively. The pharmacokinetic parameters were proportional with q12h and q8h dosing; those of the q8h regimen are shown in Table 1:
| Cmax†
(mcg/mL) | AUC‡
(mcg.h/mL) | t 1/2§(hr) | |
|---|---|---|---|
|
|||
| Quinupristin and metabolites | 3.20 ± 0.67 | 7.20 ± 1.24 | 3.07 ± 0.51 |
| Dalfopristin and metabolite | 7.96 ± 1.30 | 10.57 ± 2.24 | 1.04 ± 0.20 |
The clearances of unchanged quinupristin and dalfopristin are similar (0.72 L/h/kg), and the steady-state volume of distribution for quinupristin is 0.45 L/kg and for dalfopristin is 0.24 L/kg. The elimination half-life of quinupristin and dalfopristin is approximately 0.85 and 0.70 hours, respectively.
The total protein binding of quinupristin is higher than that of dalfopristin. Synercid does not alter the in vitro binding of warfarin to proteins in human serum.
Penetration of unchanged quinupristin and dalfopristin in noninflammatory blister fluid corresponds to about 19% and 11% of that estimated in plasma, respectively. The penetration into blister fluid of quinupristin and dalfopristin in combination with their major metabolites was in total approximately 40% compared to that in plasma.
In vitro, the transformation of the parent drugs into their major active metabolites occurs by non-enzymatic reactions and is not dependent on cytochrome-P450 or glutathione-transferase enzyme activities.
Synercid has been shown to be a major inhibitor (in vitro inhibits 70% cyclosporin A biotransformation at 10 mcg/mL of Synercid) of the activity of cytochrome P450 3A4 isoenzyme. (See WARNINGS.)
Synercid can interfere with the metabolism of other drug products that are associated with QTc prolongation. However, electrophysiologic studies confirm that Synercid does not itself induce QTc prolongation. (See WARNINGS.)
Fecal excretion constitutes the main elimination route for both parent drugs and their metabolites (75 to 77% of dose). Urinary excretion accounts for approximately 15% of the quinupristin and 19% of the dalfopristin dose. Preclinical data in rats have demonstrated that approximately 80% of the dose is excreted in the bile and suggest that in man, biliary excretion is probably the principal route for fecal elimination.
Warnings
Drug Interactions
In vitro drug interaction studies have demonstrated that Synercid significantly inhibits cytochrome P450 3A4 metabolism of cyclosporin A, midazolam, nifedipine and terfenadine. In addition, 24 subjects given Synercid 7.5 mg/kg q8h for 2 days and 300 mg of cyclosporine on day 3 showed an increase of 63% in the AUC of cyclosporine, an increase of 30% in the Cmax of cyclosporine, a 77% increase in the t1/2 of cyclosporine, and, a decrease of 34% in the clearance of cyclosporine. Therapeutic level monitoring of cyclosporine should be performed when cyclosporine must be used concomitantly with Synercid.
It is reasonable to expect that the concomitant administration of Synercid and other drugs primarily metabolized by the cytochrome P450 3A4 enzyme system may likely result in increased plasma concentrations of these drugs that could increase or prolong their therapeutic effect and/or increase adverse reactions. (See Table below.) Therefore, coadministration of Synercid with drugs which are cytochrome P450 3A4 substrates and possess a narrow therapeutic window requires caution and monitoring of these drugs (e.g., cyclosporine), whenever possible. Concomitant medications metabolized by the cytochrome P450 3A4 enzyme system that may prolong the QTc interval should be avoided.
Concomitant administration of Synercid and nifedipine (repeated oral doses) and midazolam (intravenous bolus dose) in healthy volunteers led to elevated plasma concentrations of these drugs. The Cmax increased by 18% and 14% (median values) and the AUC increased by 44% and 33% for nifedipine and midazolam, respectively.
|
| Antihistamines: astemizole, terfenadine |
| Anti-HIV (NNRTIs and Protease inhibitors): delavirdine, nevirapine, indinavir, ritonavir |
| Antineoplastic agents: vinca alkaloids (e.g., vinblastine), docetaxel, paclitaxel |
| Benzodiazepines: midazolam, diazepam |
| Calcium channel blockers: dihydropyridines (e.g., nifedipine), verapamil, diltiazem |
| Cholesterol-lowering agents: HMG-CoA reductase inhibitors (e.g., lovastatin) |
| GI motility agents: cisapride |
| Immunosuppressive agents: cyclosporine, tacrolimus |
| Steroids: methylprednisolone |
| Other: carbamazepine, quinidine, lidocaine, disopyramide |
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Synercid, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Precautions
Hepatic Insufficiency
Following a single 1-hour infusion of Synercid (7.5 mg/kg) to patients with hepatic insufficiency, plasma concentrations were significantly increased. (See CLINICAL PHARMACOLOGY: Special Populations.) However, the effect of dose reduction or increase in dosing interval on the pharmacokinetics of Synercid in these patients has not been studied. Therefore, no recommendations can be made at this time regarding the appropriate dose modification.
Pediatric Use
Synercid has been used in a limited number of pediatric patients under emergency-use conditions at a dose of 7.5 mg/kg q8h or q12h. However, the safety and effectiveness of Synercid in patients under 16 years of age have not been established.
Geriatric Use
In phase 3 comparative trials of Synercid, 37% of patients (n=404) were ≥65 years of age, of which 145 were ≥75 years of age. In the phase 3 non-comparative trials, 29% of patients (n=346) were ≥65 years of age, of which 112 were ≥75 years of age. There were no apparent differences in the frequency, type, or severity of related adverse reactions including cardiovascular events between elderly and younger individuals.
Adverse Reactions/Side Effects
The safety of Synercid was evaluated in 1099 patients enrolled in 5 comparative clinical trials. Additionally, 4 non-comparative clinical trials (3 prospective and 1 retrospective in design) were conducted in which 1199 patients received Synercid for infections due to Gram-positive pathogens for which no other treatment option was available. In non-comparative trials, the patients were severely ill, often with multiple co-morbidities or physiological impairments, and may have been intolerant to or failed other antibacterial therapies.
COMPARATIVE TRIALS
CLINICAL REACTIONS – ALL COMPARATIVE STUDIES
Adverse reactions with an incidence of ≥1% and possibly or probably related to Synercid administration include:
| Adverse Reactions | % of patients with adverse reactions | |
|---|---|---|
| Synercid | Comparator | |
| Inflammation at infusion site | 42.0 | 25.0 |
| Pain at infusion site | 40.0 | 23.7 |
| Edema at infusion site | 17.3 | 9.5 |
| Infusion site reaction | 13.4 | 10.1 |
| Nausea | 4.6 | 7.2 |
| Thrombophlebitis | 2.4 | 0.3 |
| Diarrhea | 2.7 | 3.2 |
| Vomiting | 2.7 | 3.8 |
| Rash | 2.5 | 1.4 |
| Headache | 1.6 | 0.9 |
| Pruritus | 1.5 | 1.1 |
| Pain | 1.5 | 0.1 |
Additional adverse reactions that were possibly or probably related to Synercid with an incidence less than 1% within each body system are listed below:
Body as a Whole: abdominal pain, worsening of underlying illness, allergic reaction, chest pain, fever, infection;
Cardiovascular: palpitation, phlebitis;
Digestive: constipation, dyspepsia, oral moniliasis, pancreatitis, pseudomembranous enterocolitis, stomatitis;
Metabolic: gout, peripheral edema;
Musculoskeletal: arthralgia, myalgia, myasthenia;
Nervous: anxiety, confusion, dizziness, hypertonia, insomnia, leg cramps, paresthesia, vasodilation;
Respiratory: dyspnea, pleural effusion;
Skin and Appendages: maculopapular rash, sweating, urticaria;
Urogenital: hematuria, vaginitis
CLINICAL REACTIONS – SKIN AND SKIN STRUCTURE STUDIES
In two of the five comparative clinical trials Synercid (n=450) and comparator regimens (e.g., oxacillin/vancomycin or cefazolin/vancomycin; n=443) were studied for safety and efficacy in the treatment of complicated skin and skin structure infections. The adverse event profile seen in the Synercid patients in these two studies differed significantly from that seen in the other comparative studies. What follows is safety data from these two studies.
Discontinuation of therapy was most frequently due to the following drug related events:
| % of patients discontinuing therapy by reaction type | ||
|---|---|---|
| Type | Synercid | Comparator |
| Venous | 12.0 | 2.0 |
| Non-venous | 11.8 | 4.0 |
| -Rash | 2.0 | 0.9 |
| -Nausea | 1.1 | 0.0 |
| -Vomiting | 0.9 | 0.0 |
| -Pain | 0.9 | 0.0 |
| -Pruritus | 0.9 | 0.5 |
Venous adverse events were seen predominately in patients who had peripheral infusions. The most frequently reported venous and non-venous adverse reactions possibly or probably related to study drug were:
| % of patients with adverse reactions | ||
|---|---|---|
| Synercid | Comparator | |
| Venous | 68.0 | 32.7 |
| -Pain at infusion site | 44.7 | 17.8 |
| -Inflammation at infusion site | 38.2 | 14.7 |
| -Edema at infusion site | 18.0 | 7.2 |
| -Infusion site reaction | 11.6 | 3.6 |
| Non-venous | 24.7 | 13.1 |
| -Nausea | 4.0 | 2.0 |
| -Vomiting | 3.7 | 1.0 |
| -Rash | 3.1 | 1.3 |
| -Pain | 3.1 | 0.2 |
There were eight (1.7%) episodes of thrombus or thrombophlebitis in the Synercid arms and none in the comparator arms.
Synercid Dosage and Administration
Synercid should be administered by intravenous infusion in 5% Dextrose in Water solution over a 60-minute period. (See WARNINGS.) An infusion pump or device may be used to control the rate of infusion. If necessary, central venous access (e.g., PICC) can be used to administer Synercid to decrease the incidence of venous irritation. The recommended dosage for the treatment of complicated skin and skin structure infections is 7.5 mg/kg q12h. The minimum recommended treatment duration for complicated skin and skin structure infections is seven days.
PRINCIPAL DISPLAY PANEL - 500 mg Vial Label
NDC 61570-260-01
Synercid® I.V.
(quinupristin 150mg and dalfopristin 350mg)
for Injection
500 mg
Single Dose Vial, For I.V. Use Only-Not For Direct Infusion
1 Sterile Vial
Pfizer
Injectables
Rx only

PRINCIPAL DISPLAY PANEL - 10 Vial Carton
NDC 61570-260-10
Contains 10 of NDC 61570-260-01
REFRIGERATE
Synercid® I.V.
(quinupristin 150mg and
dalfopristin 350mg) for Injection
500 mg
For I.V. Use Only. Prior to Reconstitution:
Store in a refrigerator 2–8° C (36–46° F).
10 Single Dose Vials
Pfizer
Injectables
Rx only
| SYNERCID
quinupristin and dalfopristin injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) |