Drug Detail:Tambocor (Flecainide [ flek-a-nide ])
Drug Class: Group I antiarrhythmics
Mortality. TAMBOCOR was included in the National Heart Lung and Blood Institute's Cardiac Arrhythmia Suppression Trial (CAST), a long-term, multicenter, randomized, double-blind study in patients with asymptomatic non-life-threatening ventricular arrhythmias who had a myocardial infarction more than six days but less than two years previously. An excessive mortality or non-fatal cardiac arrest rate was seen in patients treated with TAMBOCOR compared with that seen in patients assigned to a carefully matched placebo-treated group. This rate was 16/315 (5.1%) for TAMBOCOR and 7/309 (2.3%) for the matched placebo. The average duration of treatment with TAMBOCOR in this study was ten months. The applicability of the CAST results to other populations (e.g., those without recent myocardial infarction) is uncertain, but at present, it is prudent to consider the risks of Class IC agents (including TAMBOCOR), coupled with the lack of any evidence of improved survival, generally unacceptable in patients without life-threatening ventricular arrhythmias, even if the patients are experiencing unpleasant, but not life-threatening, symptoms or signs.
Ventricular Pro-arrhythmic Effects in Patients with Atrial Fibrillation/Flutter. A review of the world literature revealed reports of 568 patients treated with oral TAMBOCOR for paroxysmal atrial fibrillation/flutter (PAF). Ventricular tachycardia was experienced in 0.4% (2/568) of these patients. Of 19 patients in the literature with chronic atrial fibrillation (CAF), 10.5% (2) experienced VT or VF. FLECAINIDE IS NOT RECOMMENDED FOR USE IN PATIENTS WITH CHRONIC ATRIAL FIBRILLATION. Case reports of ventricular proarrhythmic effects in patients treated with TAMBOCOR for atrial fibrillation/flutter have included increased PVCs, VT, ventricular fibrillation (VF), and death.
As with other Class I agents, patients treated with TAMBOCOR for atrial flutter have been reported with 1:1 atrioventricular conduction due to slowing the atrial rate. A paradoxical increase in the ventricular rate also may occur in patients with atrial fibrillation who receive TAMBOCOR. Concomitant negative chronotropic therapy such as digoxin or beta-blockers may lower the risk of this complication.
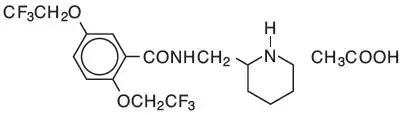
Tambocor - Clinical Pharmacology
TAMBOCOR has local anesthetic activity and belongs to the membrane stabilizing (Class 1) group of antiarrhythmic agents; it has electrophysiologic effects characteristic of the IC class of antiarrhythmics.
Precautions
Drug Interactions
TAMBOCOR has been administered to patients receiving digitalis preparations or beta-adrenergic blocking agents without adverse effects. During administration of multiple oral doses of TAMBOCOR to healthy subjects stabilized on a maintenance dose of digoxin, a 13%–19% increase in plasma digoxin levels occurred at six hours postdose.
In a study involving healthy subjects receiving TAMBOCOR and propranolol concurrently, plasma flecainide levels were increased about 20% and propranolol levels were increased about 30% compared to control values. In this formal interaction study, TAMBOCOR and propranolol were each found to have negative inotropic effects; when the drugs were administered together, the effects were additive. The effects of concomitant administration of TAMBOCOR and propranolol on the PR interval were less than additive. In TAMBOCOR clinical trials, patients who were receiving beta blockers concurrently did not experience an increased incidence of side effects. Nevertheless, the possibility of additive negative inotropic effects of beta blockers and flecainide should be recognized.
Flecainide is not extensively bound to plasma proteins. In vitro studies with several drugs which may be administered concomitantly showed that the extent of flecainide binding to human plasma proteins is either unchanged or only slightly less. Consequently, interactions with other drugs which are highly protein bound (e.g., anticoagulants) would not be expected. TAMBOCOR has been used in a large number of patients receiving diuretics without apparent interaction. Limited data in patients receiving known enzyme inducers (phenytoin, phenobarbital, carbamazepine) indicate only a 30% increase in the rate of flecainide elimination. In healthy subjects receiving cimetidine (1 gm daily) for one week, plasma flecainide levels increased by about 30% and half-life increased by about 10%.
When amiodarone is added to flecainide therapy, plasma flecainide levels may increase two-fold or more in some patients, if flecainide dosage is not reduced. (See Dosage and Administration.)
Drugs that inhibit cytochrome P450IID6, such as quinidine, might increase the plasma concentrations of flecainide in patients that are on chronic flecainide therapy; especially if these patients are extensive metabolizers.
There has been little experience with the coadministration of TAMBOCOR and either disopyramide or verapamil. Because both of these drugs have negative inotropic properties and the effects of coadministration with TAMBOCOR are unknown, neither disopyramide nor verapamil should be administered concurrently with TAMBOCOR unless, in the judgment of the physician, the benefits of this combination outweigh the risks. There has been too little experience with the coadministration of TAMBOCOR with nifedipine or diltiazem to recommend concomitant use.
Adverse Reactions/Side Effects
In post-myocardial infarction patients with asymptomatic PVCs and non-sustained ventricular tachycardia, TAMBOCOR therapy was found to be associated with a 5.1% rate of death and non-fatal cardiac arrest, compared with a 2.3% rate in a matched placebo group. (See Warnings.)
Adverse effects reported for TAMBOCOR, described in detail in the Warnings section, were new or worsened arrhythmias which occurred in 1% of 108 patients with PSVT and in 7% of 117 patients with PAF; and new or exacerbated ventricular arrhythmias which occurred in 7% of 1330 patients with PVCs, non-sustained or sustained VT. In patients treated with flecainide for sustained VT, 80% (51/64) of proarrhythmic events occurred within 14 days of the onset of therapy. 198 patients with sustained VT experienced a 13% incidence of new or exacerbated ventricular arrhythmias when dosage was initiated at 200 mg/day with slow upward titration, and did not exceed 300 mg/day in most patients. In some patients, TAMBOCOR treatment has been associated with episodes of unresuscitatable VT or ventricular fibrillation (cardiac arrest). (See Warnings.) New or worsened CHF occurred in 6.3% of 1046 patients with PVCs, non-sustained or sustained VT. Of 297 patients with sustained VT, 9.1% experienced new or worsened CHF. New or worsened CHF was reported in 0.4% of 225 patients with supraventricular arrhythmias. There have also been instances of second- (0.5%) or third-degree (0.4%) AV block. Patients have developed sinus bradycardia, sinus pause, or sinus arrest, about 1.2% altogether (see Warnings). The frequency of most of these serious adverse events probably increases with higher trough plasma levels, especially when these trough levels exceed 1.0 µg/mL.
There have been rare reports of isolated elevations of serum alkaline phosphatase and isolated elevations of serum transaminase levels. These elevations have been asymptomatic and no cause and effect relationship with TAMBOCOR has been established. In foreign postmarketing surveillance studies, there have been rare reports of hepatic dysfunction including reports of cholestasis and hepatic failure, and extremely rare reports of blood dyscrasias. Although no cause and effect relationship has been established, it is advisable to discontinue TAMBOCOR in patients who develop unexplained jaundice or signs of hepatic dysfunction or blood dyscrasias in order to eliminate TAMBOCOR as the possible causative agent.
Incidence figures for other adverse effects in patients with ventricular arrhythmias are based on a multicenter efficacy study, utilizing starting doses of 200 mg/day with gradual upward titration to 400 mg/day. Patients were treated for an average of 4.7 months, with some receiving up to 22 months of therapy. In this trial, 5.4% of patients discontinued due to non-cardiac adverse effects.
| Adverse Effect | Incidence | Incidence by Dose During Upward Titration | ||
|---|---|---|---|---|
| All 429 Patients at Any Dose | 200 mg/Day (N=426) | 300 mg/Day (N=293) | 400 mg/Day (N=100) | |
|
||||
| Dizziness* | 18.9% | 11.0% | 10.6% | 13.0% |
| Visual Disturbances† | 15.9% | 5.4% | 12.3% | 18.0% |
| Dyspnea | 10.3% | 5.2% | 7.5% | 4.0% |
| Headache | 9.6% | 4.5% | 6.1% | 9.0% |
| Nausea | 8.9% | 4.9% | 4.8% | 6.0% |
| Fatigue | 7.7% | 4.5% | 4.4% | 3.0% |
| Palpitation | 6.1% | 3.5% | 2.4% | 7.0% |
| Chest Pain | 5.4% | 3.1% | 3.8% | 1.0% |
| Asthenia | 4.9% | 2.6% | 2.0% | 4.0% |
| Tremor | 4.7% | 2.4% | 3.4% | 2.0% |
| Constipation | 4.4% | 2.8% | 2.1% | 1.0% |
| Edema | 3.5% | 1.9% | 1.4% | 2.0% |
| Abdominal Pain | 3.3% | 1.9% | 2.4% | 1.0% |
The following additional adverse experiences, possibly related to TAMBOCOR therapy and occurring in 1% to less than 3% of patients, have been reported in acute and chronic studies: Body as a Whole – malaise, fever; Cardiovascular – tachycardia, sinus pause or arrest; Gastrointestinal – vomiting, diarrhea, dyspepsia, anorexia; Skin – rash; Visual – diplopia; Nervous System – hypoesthesia, paresthesia, paresis, ataxia, flushing, increased sweating, vertigo, syncope, somnolence, tinnitus; Psychiatric – anxiety, insomnia, depression.
The following additional adverse experiences, possibly related to TAMBOCOR, have been reported in less than 1% of patients: Body as a Whole – swollen lips, tongue and mouth; arthralgia, bronchospasm, myalgia; Cardiovascular – angina pectoris, second-degree and third-degree AV block, bradycardia, hypertension, hypotension; Gastrointestinal – flatulence; Urinary System – polyuria, urinary retention; Hematologic – leukopenia, granulocytopenia, thrombocytopenia; Skin – urticaria, exfoliative dermatitis, pruritis, alopecia; Visual – eye pain or irritation, photophobia, nystagmus; Nervous System – twitching, weakness, change in taste, dry mouth, convulsions, impotence, speech disorder, stupor, neuropathy; Respiratory – pneumonitis/pulmonary infiltration possibly due to chronic flecainide treatment; Psychiatric – amnesia, confusion, decreased libido, depersonalization, euphoria, morbid dreams, apathy.
For patients with supraventricular arrhythmias, the most commonly reported noncardiac adverse experiences remain consistent with those known for patients treated with TAMBOCOR for ventricular arrhythmias. Dizziness is possibly more frequent in PAF patients.
Tambocor Dosage and Administration
For patients with sustained VT, no matter what their cardiac status, TAMBOCOR, like other antiarrhythmics, should be initiated in-hospital with rhythm monitoring.
Flecainide has a long half-life (12 to 27 hours in patients). Steady-state plasma levels, in patients with normal renal and hepatic function, may not be achieved until the patient has received 3 to 5 days of therapy at a given dose. Therefore, increases in dosage should be made no more frequently than once every four days, since during the first 2 to 3 days of therapy the optimal effect of a given dose may not be achieved.
For patients with PSVT and patients with PAF the recommended starting dose is 50 mg every 12 hours. TAMBOCOR doses may be increased in increments of 50 mg bid every four days until efficacy is achieved. For PAF patients, a substantial increase in efficacy without a substantial increase in discontinuations for adverse experiences may be achieved by increasing the TAMBOCOR dose from 50 to 100 mg bid. The maximum recommended dose for patients with paroxysmal supraventricular arrhythmias is 300 mg/day.
For sustained VT the recommended starting dose is 100 mg every 12 hours. This dose may be increased in increments of 50 mg bid every four days until efficacy is achieved. Most patients with sustained VT do not require more than 150 mg every 12 hours (300 mg/day) and the maximum dose recommended is 400 mg/day.
In patients with sustained VT, use of higher initial doses and more rapid dosage adjustments have resulted in an increased incidence of proarrhythmic events and CHF, particularly during the first few days of dosing (see Warnings). Therefore, a loading dose is not recommended.
Intravenous lidocaine has been used occasionally with TAMBOCOR while awaiting the therapeutic effect of TAMBOCOR. No adverse drug interactions were apparent. However, no formal studies have been performed to demonstrate the usefulness of this regimen.
An occasional patient not adequately controlled by (or intolerant to) a dose given at 12-hour intervals may be dosed at eight-hour intervals.
Once adequate control of the arrhythmia has been achieved, it may be possible in some patients to reduce the dose as necessary to minimize side effects or effects on conduction. In such patients, efficacy at the lower dose should be evaluated.
TAMBOCOR should be used cautiously in patients with a history of CHF or myocardial dysfunction (see Warnings).
Any use of TAMBOCOR in children should be directly supervised by a cardiologist skilled in the treatment of arrhythmias in children. Because of the evolving nature of information in this area, specialized literature should be consulted. Under six months of age, the initial starting dose of TAMBOCOR in children is approximately 50 mg/M2 body surface area daily, divided into two or three equally spaced doses. Over six months of age, the initial starting dose may be increased to 100 mg/M2 per day. The maximum recommended dose is 200 mg/M2 per day. This dose should not be exceeded. In some children on higher doses, despite previously low plasma levels, the level has increased rapidly to far above therapeutic values while taking the same dose. Small changes in dose may also lead to disproportionate increases in plasma levels. Plasma trough (less than one hour pre-dose) flecainide levels and electrocardiograms should be obtained at presumed steady state (after at least five doses) either after initiation or change in TAMBOCOR dose, whether the dose was increased for lack of effectiveness, or increased growth of the patient. For the first year on therapy, whenever the patient is seen for reasons of clinical follow-up, it is suggested that a 12-lead electrocardiogram and plasma trough flecainide level are obtained. The usual therapeutic level of flecainide in children is 200–500 ng/mL. In some cases, levels as high as 800 ng/mL may be required for control.
In patients with severe renal impairment (creatinine clearance of 35 mL/min/1.73 square meters or less), the initial dosage should be 100 mg once daily (or 50 mg bid); when used in such patients, frequent plasma level monitoring is required to guide dosage adjustments (see Plasma Level Monitoring). In patients with less severe renal disease, the initial dosage should be 100 mg every 12 hours; plasma level monitoring may also be useful in these patients during dosage adjustment. In both groups of patients, dosage increases should be made very cautiously when plasma levels have plateaued (after more than four days), observing the patient closely for signs of adverse cardiac effects or other toxicity. It should be borne in mind that in these patients it may take longer than four days before a new steady-state plasma level is reached following a dosage change.
Based on theoretical considerations, rather than experimental data, the following suggestion is made: when transferring patients from another antiarrhythmic drug to TAMBOCOR allow at least two to four plasma half-lives to elapse for the drug being discontinued before starting TAMBOCOR at the usual dosage. In patients where withdrawal of a previous antiarrhythmic agent is likely to produce life-threatening arrhythmias, the physician should consider hospitalizing the patient.
When flecainide is given in the presence of amiodarone, reduce the usual flecainide dose by 50% and monitor the patient closely for adverse effects. Plasma level monitoring is strongly recommended to guide dosage with such combination therapy (see below).
Plasma Level Monitoring
The large majority of patients successfully treated with TAMBOCOR were found to have trough plasma levels between 0.2 and 1.0 µg/mL. The probability of adverse experiences, especially cardiac, may increase with higher trough plasma levels, especially when these exceed 1.0 µg/mL. Periodic monitoring of trough plasma levels may be useful in patient management. Plasma level monitoring is required in patients with severe renal failure or severe hepatic disease, since elimination of flecainide from plasma may be markedly slower. Monitoring of plasma levels is strongly recommended in patients on concurrent amiodarone therapy and may also be helpful in patients with CHF and in patients with moderate renal disease.
| TAMBOCOR
flecainide acetate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| TAMBOCOR
flecainide acetate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| TAMBOCOR
flecainide acetate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Medicis Pharmaceutical Corp (182837492) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| 3M Drug Delivery Systems | 128688199 | MANUFACTURE(99207-180, 99207-181, 99207-182) | |