Drug Detail:Tazicef (Ceftazidime (injection) [ sef-tay-zi-deem ])
Drug Class: Third generation cephalosporins
Tazicef Description
Ceftazidime is a semisynthetic, broad-spectrum, beta-lactam antibacterial drug for parenteral administration. It is the pentahydrate of pyridinium, 1-[[7-[[(2-amino-4-thiazolyl)[(1-carboxy-1-methylethoxy) imino]acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-, hydroxide, inner salt, [6R-[6α,7β(Z)]]. It has the following structure:
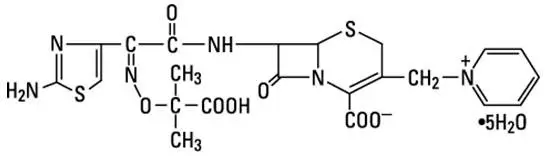
The molecular formula is C22H32N6O12S2, representing a molecular weight of 636.6.
Tazicef (ceftazidime for injection, USP) is a sterile, dry-powdered mixture of ceftazidime pentahydrate and sodium carbonate. The sodium carbonate at a concentration of 118 mg/g of ceftazidime activity has been admixed to facilitate dissolution. The total sodium content of the mixture is approximately 51 mg (2.2 mEq)/g of ceftazidime activity.
Tazicef in sterile crystalline form is supplied in ADD-Vantage® vials equivalent to 1 g or 2 g of anhydrous ceftazidime.
Solutions of Tazicef range in color from light yellow to amber, depending on the diluent and volume used. The pH of freshly constituted solutions usually ranges from 5 to 7.5.
Tazicef - Clinical Pharmacology
After IV administration of 500-mg and 1-g doses of ceftazidime over 5 minutes to normal adult male volunteers, mean peak serum concentrations of 45 and 90 mcg/mL, respectively, were achieved. After IV infusion of 500-mg, 1-g, and 2-g doses of ceftazidime over 20 to 30 minutes to normal adult male volunteers, mean peak serum concentrations of 42, 69, and 170 mcg/mL, respectively, were achieved. The average serum concentrations following IV infusion of 500-mg, 1-g, and 2-g doses to these volunteers over an 8-hour interval are given in Table 1.
| Ceftazidime | Serum Concentrations (mcg/mL) | ||||
|---|---|---|---|---|---|
| IV Dose | 0.5 hr | 1 hr | 2 hr | 4 hr | 8 hr |
| 500 mg | 42 | 25 | 12 | 6 | 2 |
| 1 g | 60 | 39 | 23 | 11 | 3 |
| 2 g | 129 | 75 | 42 | 13 | 5 |
The absorption and elimination of ceftazidime were directly proportional to the size of the dose. The half-life following IV administration was approximately 1.9 hours. Less than 10% of ceftazidime was protein bound. The degree of protein binding was independent of concentration.
There was no evidence of accumulation of ceftazidime in the serum in individuals with normal renal function following multiple IV doses of 1 and 2 g every 8 hours for 10 days.
Following intramuscular (IM) administration of 500-mg and 1-g doses of ceftazidime to normal adult volunteers, the mean peak serum concentrations were 17 and 39 mcg/mL, respectively, at approximately 1 hour. Serum concentrations remained above 4 mcg/mL for 6 and 8 hours after the IM administration of 500-mg and 1-g doses, respectively. The half-life of ceftazidime in these volunteers was approximately 2 hours.
The presence of hepatic dysfunction had no effect on the pharmacokinetics of ceftazidime in individuals administered 2 g intravenously every 8 hours for 5 days. Therefore, a dosage adjustment from the normal recommended dosage is not required for patients with hepatic dysfunction, provided renal function is not impaired.
Approximately 80% to 90% of an IM or IV dose of ceftazidime is excreted unchanged by the kidneys over a 24-hour period. After the IV administration of single 500-mg or 1-g doses, approximately 50% of the dose appeared in the urine in the first 2 hours. An additional 20% was excreted between 2 and 4 hours after dosing, and approximately another 12% of the dose appeared in the urine between 4 and 8 hours later. The elimination of ceftazidime by the kidneys resulted in high therapeutic concentrations in the urine.
The mean renal clearance of ceftazidime was approximately 100 mL/min. The calculated plasma clearance of approximately 115 mL/min indicated nearly complete elimination of ceftazidime by the renal route. Administration of probenecid before dosing had no effect on the elimination kinetics of ceftazidime. This suggested that ceftazidime is eliminated by glomerular filtration and is not actively secreted by renal tubular mechanisms.
Since ceftazidime is eliminated almost solely by the kidneys, its serum half-life is significantly prolonged in patients with impaired renal function. Consequently, dosage adjustments in such patients as described in the DOSAGE AND ADMINISTRATION section are suggested.
Therapeutic concentrations of ceftazidime are achieved in the following body tissues and fluids.
| Tissue or Fluid | Dose/Route | No. of Patients | Time of Sample Post Dose | Average Tissue or Fluid Level (mcg/mL or mcg/g) |
|---|---|---|---|---|
| Urine | 500 mg IM | 6 | 0 to 2 hr | 2,100 |
| 2 g IV | 6 | 0 to 2 hr | 12,000 | |
| Bile | 2 g IV | 3 | 90 min | 36.4 |
| Synovial fluid | 2 g IV | 13 | 2 hr | 25.6 |
| Peritoneal fluid | 2 g IV | 8 | 2 hr | 48.6 |
| Sputum | 1 g IV | 8 | 1 hr | 9 |
| Cerebrospinal fluid | 2 g q8hr IV | 5 | 120 min | 9.8 |
| (inflamed meninges) | 2 g q8hr IV | 6 | 180 min | 9.4 |
| Aqueous humor | 2 g IV | 13 | 1 to 3 hr | 11 |
| Blister fluid | 1 g IV | 7 | 2 to 3 hr | 19.7 |
| Lymphatic fluid | 1 g IV | 7 | 2 to 3 hr | 23.4 |
| Bone | 2 g IV | 8 | 0.67 hr | 31.1 |
| Heart muscle | 2 g IV | 35 | 30 to 280 min | 12.7 |
| Skin | 2 g IV | 22 | 30 to 180 min | 6.6 |
| Skeletal muscle | 2 g IV | 35 | 30 to 280 min | 9.4 |
| Myometrium | 2 g IV | 31 | 1 to 2 hr | 18.7 |
Related/similar drugs
prednisone, amoxicillin, doxycycline, ciprofloxacin, cephalexin, metronidazole, azithromycinIndications and Usage for Tazicef
Tazicef (ceftazidime for injection, USP) is indicated for the treatment of patients with infections caused by susceptible strains of the designated organisms in the following diseases:
- 1.
- Lower Respiratory Tract Infections, including pneumonia, caused by Pseudomonas aeruginosa and other Pseudomonas spp.; Haemophilus influenzae, including ampicillin-resistant strains; Klebsiella spp.; Enterobacter spp.; Proteus mirabilis; Escherichia coli; Serratia spp.; Citrobacter spp.; Streptococcus pneumoniae; and Staphylococcus aureus (methicillin-susceptible strains).
- 2.
- Skin and Skin-Structure Infections caused by Pseudomonas aeruginosa; Klebsiella spp.; Escherichia coli; Proteus spp., including Proteus mirabilis and indole-positive Proteus; Enterobacter spp.; Serratia spp.; Staphylococcus aureus (methicillin-susceptible strains); and Streptococcus pyogenes (group A beta-hemolytic streptococci).
- 3.
- Urinary Tract Infections, both complicated and uncomplicated, caused by Pseudomonas aeruginosa; Enterobacter spp.; Proteus spp., including Proteus mirabilis and indole-positive Proteus; Klebsiella spp.; and Escherichia coli.
- 4.
- Bacterial Septicemia caused by Pseudomonas aeruginosa, Klebsiella spp., Haemophilus influenzae, Escherichia coli, Serratia spp., Streptococcus pneumoniae, and Staphylococcus aureus (methicillin-susceptible strains).
- 5.
- Bone and Joint Infections caused by Pseudomonas aeruginosa, Klebsiella spp., Enterobacter spp., and Staphylococcus aureus (methicillin-susceptible strains).
- 6.
- Gynecologic Infections, including endometritis, pelvic cellulitis, and other infections of the female genital tract caused by Escherichia coli.
- 7.
- Intra-abdominal Infections, including peritonitis caused by Escherichia coli, Klebsiella spp., and Staphylococcus aureus (methicillin-susceptible strains) and polymicrobial infections caused by aerobic and anaerobic organisms and Bacteroides spp. (many strains of Bacteroides fragilis are resistant).
- 8.
- Central Nervous System Infections, including meningitis, caused by Haemophilus influenzae and Neisseria meningitidis. Ceftazidime has also been used successfully in a limited number of cases of meningitis due to Pseudomonas aeruginosa and Streptococcus pneumoniae.
Tazicef (ceftazidime for injection, USP) may be used alone in cases of confirmed or suspected sepsis. Ceftazidime has been used successfully in clinical trials as empiric therapy in cases where various concomitant therapies with other antibacterial drugs have been used.
Tazicef (ceftazidime for injection, USP) may also be used concomitantly with other antibacterial drugs, such as aminoglycosides, vancomycin, and clindamycin; in severe and life-threatening infections; and in the immunocompromised patient. When such concomitant treatment is appropriate, prescribing information in the labeling for the other antibacterial drugs should be followed. The dose depends on the severity of the infection and the patient's condition.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Tazicef (ceftazidime for injection, USP) and other antibacterial drugs, Tazicef (ceftazidime for injection, USP) should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Warnings
BEFORE THERAPY WITH TAZICEF IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFTAZIDIME, CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-HYPERSENSITIVITY AMONG BETA-LACTAM ANTIBACTERIAL DRUGS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO TAZICEF OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, IV FLUIDS, IV ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ceftazidime, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial drug treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Elevated levels of ceftazidime in patients with renal insufficiency can lead to seizures, nonconvulsive status epilepticus (NCSE), encephalopathy, coma, asterixis, neuromuscular excitability, and myoclonia (see PRECAUTIONS).
Precautions
General
High and prolonged serum ceftazidime concentrations can occur from usual dosages in patients with transient or persistent reduction of urinary output because of renal insufficiency. The total daily dosage should be reduced when ceftazidime is administered to patients with renal insufficiency (see DOSAGE AND ADMINISTRATION). Elevated levels of ceftazidime in these patients can lead to seizures, nonconvulsive status epilepticus, encephalopathy, coma, asterixis, neuromuscular excitability, and myoclonia. Continued dosage should be determined by degree of renal impairment, severity of infection, and susceptibility of the causative organisms.
As with other antibacterial drugs, prolonged use of Tazicef (ceftazidime for injection, USP) may result in overgrowth of nonsusceptible organisms. Repeated evaluation of the patient's condition is essential. If superinfection occurs during therapy, appropriate measures should be taken.
Inducible type I beta-lactamase resistance has been noted with some organisms (e.g., Enterobacter spp., Pseudomonas spp., and Serratia spp.). As with other extended-spectrum beta-lactam antibacterial drugs, resistance can develop during therapy, leading to clinical failure in some cases. When treating infections caused by these organisms, periodic susceptibility testing should be performed when clinically appropriate. If patients fail to respond to monotherapy, an aminoglycoside or similar agent should be considered.
Cephalosporins may be associated with a fall in prothrombin activity. Those at risk include patients with renal and hepatic impairment, or poor nutritional state, as well as patients receiving a protracted course of antimicrobial therapy. Prothrombin time should be monitored in patients at risk and exogenous vitamin K administered as indicated.
Tazicef should be prescribed with caution in individuals with a history of gastrointestinal disease, particularly colitis.
Distal necrosis can occur after inadvertent intra-arterial administration of ceftazidime.
Prescribing Tazicef (ceftazidime) in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Adverse Reactions/Side Effects
Ceftazidime is generally well tolerated. The incidence of adverse reactions associated with the administration of ceftazidime was low in clinical trials. The most common were local reactions following IV injection and allergic and gastrointestinal reactions. Other adverse reactions were encountered infrequently. No disulfiram-like reactions were reported.
The following adverse effects from clinical trials were considered to be either related to ceftazidime therapy or were of uncertain etiology:
Local Effects, reported in fewer than 2% of patients, were phlebitis and inflammation at the site of injection (1 in 69 patients).
Hypersensitivity Reactions, reported in 2% of patients, were pruritus, rash, and fever. Immediate reactions, generally manifested by rash and/or pruritus, occurred in 1 in 285 patients. Toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme have also been reported with cephalosporin antibacterial drugs, including ceftazidime. Angioedema and anaphylaxis (bronchospasm and/or hypotension) have been reported very rarely.
Gastrointestinal Symptoms, reported in fewer than 2% of patients, were diarrhea (1 in 78), nausea (1 in 156), vomiting (1 in 500), and abdominal pain (1 in 416). The onset of pseudomembranous colitis symptoms may occur during or after treatment (see WARNINGS).
Central Nervous System Reactions (fewer than 1%) included headache, dizziness, and paresthesia. Seizures have been reported with several cephalosporins, including ceftazidime. In addition, encephalopathy, coma, asterixis, neuromuscular excitability, and myoclonia have been reported in renally impaired patients treated with unadjusted dosing regimens of ceftazidime (see PRECAUTIONS: General).
Less Frequent Adverse Events (fewer than 1%) were candidiasis (including oral thrush) and vaginitis.
Tazicef Dosage and Administration
Dosage
The usual adult dosage is 1 gram administered intravenously every 8 to 12 hours. The dosage and route should be determined by the susceptibility of the causative organisms, the severity of infection, and the condition and renal function of the patient.
The guidelines for dosage of Tazicef (ceftazidime for injection, USP) are listed in Table 3. The following dosage schedule is recommended.
| Dose | Frequency | |
|---|---|---|
| Adult | ||
|
||
| Usual recommended dosage | 1 gram intravenous | every 8 to 12 hours |
| Uncomplicated urinary tract infection | 250 mg intravenous | every 12 hours |
| Bone and joint infections | 2 grams intravenous | every 12 hours |
| Complicated urinary tract infections | 500 mg intravenous | every 8 to 12 hours |
| Uncomplicated pneumonia; mild skin and skin-structure infections | 500 mg to 1 gram intravenous | every 8 hours |
| Serious gynecological and intra-abdominal infections | 2 grams intravenous | every 8 hours |
| Meningitis | 2 grams intravenous | every 8 hours |
| Very severe life-threatening infections, especially in immunocompromised patients | 2 grams intravenous | every 8 hours |
| Lung infections caused by Pseudomonas spp. in patients with cystic fibrosis with normal renal function* | 30 to 50 mg/kg intravenous to a maximum of 6 grams per day | every 8 hours |
| Neonates (0 - 4 weeks) | 30 mg/kg intravenous | every 12 hours |
| Infants and children
(1 month to 12 years) | 30 to 50 mg/kg intravenous to a maximum of 6 grams per day† | every 8 hours |
Impaired Hepatic Function
No adjustment in dosage is required for patients with hepatic dysfunction.
Impaired Renal Function
Ceftazidime is excreted by the kidneys, almost exclusively by glomerular filtration. Therefore, in patients with impaired renal function (glomerular filtration rate [GFR] <50 mL/min), it is recommended that the dosage of ceftazidime be reduced to compensate for its slower excretion. In patients with suspected renal insufficiency, an initial loading dose of 1 gram of Tazicef may be given. An estimate of GFR should be made to determine the appropriate maintenance dosage. The recommended dosage is presented in Table 4.
| NOTE: if the dose recommended in Table 3 above is lower than that recommended for patients with renal insufficiency as outlined in Table 4, the lower dose should be used. | ||
|---|---|---|
| Creatinine Clearance (mL/min) | Recommended Unit Dose of Tazicef | Frequency of Dosing |
| 50 to 31 | 1 gram | every 12 hours |
| 30 to 16 | 1 gram | every 24 hours |
| 15 to 6 | 500 mg | every 24 hours |
| less than 5 | 500 mg | every 48 hours |
When only serum creatinine is available, the following formula (Cockcroft's equation)1 may be used to estimate creatinine clearance. The serum creatinine should represent a steady state of renal function:
| Males: Creatinine clearance (mL/min) = | Weight (kg) × (140 - age) |
| 72 × serum creatinine (mg/dL) | |
| Females: 0.85 × male value |
In patients with severe infections who would normally receive 6 grams of Tazicef daily were it not for renal insufficiency, the unit dose given in the table above may be increased by 50% or the dosing frequency may be increased appropriately. Further dosing should be determined by therapeutic monitoring, severity of the infection, and susceptibility of the causative organism.
In pediatric patients as for adults, the creatinine clearance should be adjusted for body surface area or lean body mass, and the dosing frequency should be reduced in cases of renal insufficiency.
In patients undergoing hemodialysis, a loading dose of 1 gram is recommended, followed by 1 gram after each hemodialysis period.
Tazicef (ceftazidime for injection, USP) can also be used in patients undergoing intraperitoneal dialysis and continuous ambulatory peritoneal dialysis. In such patients, a loading dose of 1 gram of Tazicef may be given, followed by 500 mg every 24 hours. In addition to IV use, Tazicef can be incorporated in the dialysis fluid at a concentration of 250 mg for 2 L of dialysis fluid.
Note: Generally, Tazicef should be continued for 2 days after the signs and symptoms of infection have disappeared, but in complicated infections longer therapy may be required.
How is Tazicef supplied
Tazicef in the dry state should be stored at 20°C to 25°C (68°F to 77°F) [See USP Controlled Room Temperature] and protected from light. Tazicef (ceftazidime for injection, USP) is a dry, white to off-white powder supplied in ADD-Vantage® vials as follows:
| Unit of Sale | Concentration |
|---|---|
| NDC 0409-5092-16 | |
| 25 single-dose ADD-Vantage® vials in a carton | 1 g/vial |
| NDC 0409-5093-11 | |
| 10 single-dose ADD-Vantage® vials in a carton | 2 g/vial |
References
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31-41.
CLINITEST and CLINISTIX are registered trademarks of Ames Division, Miles Laboratories, Inc.
INSTRUCTIONS FOR USE
DIRECTIONS FOR USE OF TAZICEF® (CEFTAZIDIME FOR INJECTION, USP)
ADD-VANTAGE® VIALS:
1g, 2g
To Open Diluent Container:
Peel the corner of the ADD-Vantage® diluent overwrap and remove flexible diluent container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
To Assemble Vial and Flexible Diluent Container (Use Aseptic Technique):
- Remove the protective covers from the top of the vial and the vial port on the diluent container as follows:
- a.
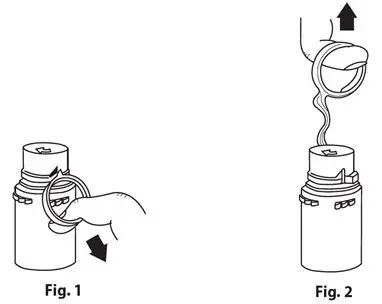
- To remove the breakaway vial cap, swing the pull ring over the top of the vial and pull down far enough to start the opening (see Figure 1), then pull straight up to remove the cap (see Figure 2).
Note: Once the breakaway cap has been removed, do not access vial with syringe. - b.
- To remove the vial port cover, grasp the tab on the pull ring, pull up to break the three tie strings, then pull back to remove the cover (see Figure 3).
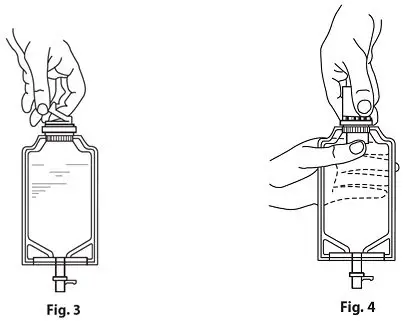
- Screw the vial into the vial port until it will go no further. THE VIAL MUST BE SCREWED IN TIGHTLY TO ASSURE A SEAL. This occurs approximately one-half turn (180°) after the first audible click (see Figure 4). The clicking sound does not assure a seal; the vial must be turned as far as it will go.
Note: Once vial is sealed, do not attempt to remove (see Figure 4). - Recheck the vial to assure that it is tight by trying to turn it further in the direction of assembly.
- Label appropriately.
To Prepare Admixture:
- Squeeze the bottom of the diluent container gently to inflate the portion of the container surrounding the end of the drug vial.
- With the other hand, push the drug vial down into the container, telescoping the walls of the container. Grasp the inner cap of the vial through the walls of the container (see Figure 5).
- Pull the inner cap from the drug vial (see Figure 6). Verify that the rubber stopper has been pulled out, allowing the drug and diluent to mix.
- Mix container contents thoroughly and use within the specified time.
Preparation for Administration (Use Aseptic Technique):
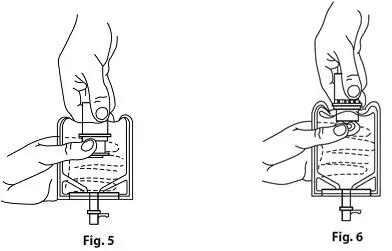
- Confirm the activation and admixture of vial contents.
- Check for leaks by squeezing container firmly. If leaks are found, discard unit as sterility may be impaired.
- Close flow control clamp of administration set.
- Remove cover from outlet port at bottom of container.
- Insert piercing pin of administration set into port with a twisting motion until the pin is firmly seated.
Note: See full directions on administration set carton. - Lift the free end of the hanger loop on the bottom of the vial, breaking the two tie strings. Bend the loop outward to lock it in the upright position, then suspend container from hanger.
- Squeeze and release drip chamber to establish proper fluid level in chamber.
- Open flow control clamp and clear air from set. Close clamp.
- Attach set to venipuncture device. If device is not indwelling, prime and make venipuncture.
- Regulate rate of administration with flow control clamp.
WARNING: Do not use flexible container in series connections.
Manufactured by Sandoz GmbH for
Hospira, Inc., Lake Forest, IL 60045, USA

LAB-1447-1.0
Revised: 12/2020
PRINCIPAL DISPLAY PANEL - 1 g Vial Carton
25 Single-dose ADD-Vantage® Vials
NDC 0409-5092-16
Contains 25 of NDC 0409-5092-11
TAZICEF®
CEFTAZIDIME FOR INJECTION, USP
equivalent to 1 g/vial ceftazidime
Rx only
For Intravenous Infusion Only
Note: For use only with ADD-Vantage® diluent container.
Hospira
| TAZICEF
ceftazidime injection, powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| TAZICEF
ceftazidime injection, powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Hospira, Inc. (141588017) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sandoz GmbH | 300220969 | ANALYSIS(0409-5092, 0409-5093) , API MANUFACTURE(0409-5092, 0409-5093) , MANUFACTURE(0409-5092, 0409-5093) , LABEL(0409-5092, 0409-5093) , PACK(0409-5092, 0409-5093) | |