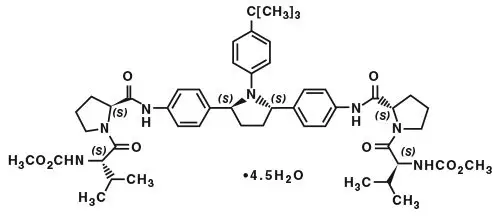
Drug Detail:Technivie (Ombitasvir, paritaprevir, and ritonavir [ om-bit-as-vir, par-i-ta-pre-vir, and-ri-toe-na-vir ])
Drug Class: Antiviral combinations
Highlights of Prescribing Information
TECHNIVIE (ombitasvir, paritaprevir and ritonavir) tablets, for oral use
Initial U.S. Approval: 2015
WARNING: RISK OF HEPATITIS B VIRUS REACTIVATION IN PATIENTS COINFECTED WITH HCV AND HBV
See full prescribing information for complete boxed warning.
Hepatitis B virus (HBV) reactivation has been reported, in some cases resulting in fulminant hepatitis, hepatic failure, and death. (5.1)
Recent Major Changes
Indications and Usage for Technivie
TECHNIVIE is a fixed-dose combination of ombitasvir, a hepatitis C virus NS5A inhibitor, paritaprevir, a hepatitis C virus NS3/4A protease inhibitor, and ritonavir, a CYP3A inhibitor and is indicated in combination with ribavirin for the treatment of patients with genotype 4 chronic hepatitis C virus (HCV) infection without cirrhosis or with compensated cirrhosis. (1)
Technivie Dosage and Administration
Testing Prior to the Initiation of Therapy:
- Test all patients for HBV infection by measuring HBsAg and anti-HBc. (2.1)
- Assess hepatic laboratory and clinical evidence of hepatic decompensation. Prior to initiation of ribavirin, assess for underlying cardiac disease. (2.1)
- Recommended dosage: Two tablets taken orally once daily (in the morning) with a meal without regard to fat or calorie content. TECHNIVIE is recommended to be used in combination with ribavirin. (2.2)
| Patient Population | Treatment | Duration |
| Genotype 4 without cirrhosis
or with compensated cirrhosis | TECHNIVIE + ribavirin* | 12 weeks |
| *TECHNIVIE administered without ribavirin for 12 weeks may be considered for treatment-naïve patients without cirrhosis who cannot take or tolerate ribavirin [see Microbiology (12.4) and Clinical Studies (14)]. | ||
Dosage Forms and Strengths
Tablets: 12.5 mg ombitasvir, 75 mg paritaprevir, 50 mg ritonavir. (3)
Contraindications
- The contraindications to ribavirin also apply to this combination regimen. (4)
- Patients with moderate to severe hepatic impairment. (4, 5.2, 8.6, 12.3)
- Co-administration with drugs that are: highly dependent on CYP3A for clearance; moderate and strong inducers of CYP3A. (4)
- Known hypersensitivity to ritonavir (e.g. toxic epidermal necrolysis, Stevens-Johnson syndrome). (4)
Warnings and Precautions
-
Risk of Hepatitis B Virus Reactivation:
Test all patients for evidence of current or prior HBV infection before initiation of HCV treatment. Monitor HCV/HBV coinfected patients for HBV reactivation and hepatitis flare during HCV treatment and post-treatment follow-up. Initiate appropriate patient management for HBV infection as clinically indicated. (5.1)
- Hepatic Decompensation and Hepatic Failure in Patient with Cirrhosis: Hepatic decompensation and hepatic failure, including liver transplantation or fatal outcomes, have been reported mostly in patients with cirrhosis. Monitor for clinical signs and symptoms of hepatic decompensation, and discontinue treatment in patients who develop evidence of hepatic decompensation. (5.2)
- ALT Elevations: Discontinue ethinyl estradiol-containing medications prior to starting TECHNIVIE (alternative contraceptive methods are recommended). Perform hepatic laboratory testing on all patients during the first 4 weeks of treatment. For ALT elevations on TECHNIVIE, monitor closely and follow recommendations in full prescribing information. (5.3)
- Risks Associated With Ribavirin Combination Treatment: The warnings and precautions for ribavirin also apply to this combination regimen. (5.4)
- Drug Interactions: The concomitant use of TECHNIVIE and certain other drugs may result in known or potentially significant drug interactions, some of which may lead to loss of therapeutic effect of TECHNIVIE. (5.5)
Adverse Reactions/Side Effects
The most commonly reported adverse reactions (incidence greater than 10% of subjects, all grades) observed with treatment with ombitasvir, paritaprevir and ritonavir with ribavirin for 12 weeks in patients without cirrhosis were asthenia, fatigue, nausea and insomnia. (6.1)
The most common adverse events (incidence greater than 10% of subjects, all grades) observed with treatment with TECHNIVIE and ribavirin for 12 weeks in patients in compensated cirrhosis were fatigue, asthenia, headache, musculoskeletal pain, pruritus, insomnia/sleep disorder, skin reactions, mood disorders, nausea, dizziness and dyspnea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AbbVie Inc. at 1-800-633-9110 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Co-administration of TECHNIVIE can alter the plasma concentrations of some drugs and some drugs may alter the plasma concentrations of TECHNIVIE. The potential for drug-drug interactions must be considered before and during treatment. Consult the full prescribing information prior to and during treatment for potential drug interactions. (4, 5.5, 7, 12.3)
- Clearance of HCV infection with direct-acting antivirals may lead to changes in hepatic function, which may impact safe and effective use of concomitant medications. Frequent monitoring of relevant laboratory parameters (INR or blood glucose) and dose adjustments of certain concomitant medications may be necessary. (7.3)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2019
Related/similar drugs
Epclusa, Mavyret, Harvoni, sofosbuvir / velpatasvir, Sovaldi, VoseviFull Prescribing Information
2. Technivie Dosage and Administration
2.1 Testing Prior to the Initiation of Therapy
- Test all patients for evidence of current or prior HBV infection by measuring hepatitis B surface antigen (HBsAg) and hepatitis B core antibody (anti-HBc) before initiating HCV treatment with TECHNIVIE [see Warnings and Precautions (5.1)].
- Prior to initiation of TECHNIVIE, assess hepatic laboratory and clinical evidence of hepatic decompensation. Prior to initiation of ribavirin, assess for underlying cardiac disease and refer to the ribavirin prescribing information [see Contraindications (4) and Warnings and Precautions (5.1 and 5.2)].
2.2 Recommended Dosage in Adults
TECHNIVIE is ombitasvir, paritaprevir and ritonavir fixed dose combination tablets.
| Patient Population | Treatment | Duration |
| Genotype 4 without cirrhosis or with compensated cirrhosis (Child-Pugh A) | TECHNIVIE + ribavirin* | 12 weeks |
| *TECHNIVIE administered without RBV for 12 weeks may be considered for treatment-naïve patients without cirrhosis who cannot take or tolerate ribavirin [see Microbiology (12.4) and Clinical Studies (14)]. | ||
4. Contraindications
- The contraindications to ribavirin also apply to this combination regimen. Refer to the ribavirin prescribing information for a list of contraindications for ribavirin.
- TECHNIVIE is contraindicated:
- In patients with moderate to severe hepatic impairment (Child-Pugh B and C) due to risk of potential toxicity [see Warnings and Precautions (5.2), Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
- With drugs that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events [see Drug Interactions (7) and Clinical Pharmacology (12.3)]:
- Alpha1-adrenoreceptor antagonist: alfuzosin HCL
- Anti-anginal: ranolazine
- Antiarrhythmic: dronedarone
- Anti-gout: colchicine in patients with renal and/or hepatic impairment
- Antipsychotic: lurasidone, pimozide
- Ergot derivatives: ergotamine, dihydroergotamine, methylergonovine
- Ethinyl estradiol-containing products such as combined oral contraceptives
- GI Motility Agent: cisapride
- HMG-CoA Reductase Inhibitors: atorvastatin, lovastatin, simvastatin
- Immunosuppressants: everolimus, sirolimus, tacrolimus
- Microsomal triglyceride transfer protein inhibitor: lomitapide
- Non-nucleoside reverse transcriptase inhibitor: efavirenz
- Phosphodiesterase-5(PDE5) inhibitor: sildenafil when dosed as Revatio for the treatment of pulmonary arterial hypertension (PAH)
- Sedatives/hypnotics: triazolam, orally administered midazolam
- With drugs that are moderate or strong inducers of CYP3A and may lead to reduced efficacy of TECHNIVIE [see Drug Interactions (7) and Clinical Pharmacology (12.3)]:
- Anticonvulsants: carbamazepine, phenytoin, phenobarbital
- Androgen receptor inhibitor: apalutamide
- Antimycobacterial: rifampin
- Herbal Product: St. John’s Wort (Hypericum perforatum)
- In patients with known hypersensitivity to ritonavir (e.g. toxic epidermal necrolysis (TEN) or Stevens-Johnson syndrome).
5. Warnings and Precautions
5.2 Risk of Hepatic Decompensation and Hepatic Failure in Patients with Cirrhosis
For patients with compensated cirrhosis:
- Monitor for clinical signs and symptoms of hepatic decompensation (such as ascites, hepatic encephalopathy, variceal hemorrhage).
- Hepatic laboratory testing including direct bilirubin levels should be performed at baseline and during the first 4 weeks of starting treatment and as clinically indicated.
- Discontinue TECHNIVIE in patients who develop evidence of hepatic decompensation.
5.3 Increased Risk of ALT Elevations
- Patients should be instructed to consult their health care professional without delay if they have onset of fatigue, weakness, lack of appetite, nausea and vomiting, jaundice or discolored feces.
- Consider discontinuing TECHNIVIE if ALT levels remain persistently greater than 10 times the ULN.
- Discontinue TECHNIVIE if ALT elevation is accompanied by signs or symptoms of liver inflammation or increasing direct bilirubin, alkaline phosphatase, or INR.
5.5 Risk of Adverse Reactions or Reduced Therapeutic Effect Due to Drug Interactions
- Loss of therapeutic effect of TECHNIVIE and possible development of resistance
- Possible clinically significant adverse reactions from greater exposures of concomitant drugs or components of TECHNIVIE.
6. Adverse Reactions/Side Effects
The following adverse reaction is described below and elsewhere in the labeling:
- Risk of Hepatic Decompensation and Hepatic Failure in Patients with Cirrhosis [see Warnings and Precautions (5.2)]
- Increased Risk of ALT Elevations [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
| PEARL-I Without Cirrhosis |
||
| Adverse Reaction | Ombitasvir, paritaprevir, ritonavir + RBV N = 91 % | Ombitasvir, paritaprevir, ritonavir N = 44 % |
| Asthenia | 29 | 25 |
| Fatigue | 15 | 7 |
| Nausea | 14 | 9 |
| Insomnia | 13 | 5 |
| Pruritus1 | 7 | 5 |
| Skin reactions2,3 | 7 | 5 |
| 1Grouped term ‘pruritus’ includes the preferred terms pruritus and pruritus generalized. 2Grouped term ‘skin reactions’ includes the preferred terms rash, erythema, eczema, rash maculo-papular, rash macular, dermatitis, rash papular, skin exfoliation, rash pruritic, rash erythematous, rash generalized, dermatitis allergic, dermatitis contact, exfoliative rash, photosensitivity reaction, psoriasis, skin reaction, ulcer and urticaria. 3The majority of events were graded as mild in severity. |
||
| AGATE-I Compensated Cirrhosis |
|
| Adverse Events | TECHNIVIE + RBV N=120 (%) |
| Fatigue | 25 |
| Asthenia | 25 |
| Headache | 23 |
| Musculoskeletal Pain/Changes1 | 17 |
| Pruritus | 16 |
| Insomnia/Sleep Disorder2 | 14 |
| Skin Reactions3 | 13 |
| Dyspnea4 | 11 |
| Mood Disorders5 | 11 |
| Nausea | 11 |
| Dizziness | 11 |
| Cardiac Events6 | 9 |
| Abdominal Pain7 | 9 |
| Cough | 7 |
| Clinical Liver or Bilirubin Related Events8 | 7 |
| Edema9 | 6 |
| Altered Mental Status10 | 6 |
| Decreased Appetite | 6 |
| Vomiting | 6 |
| 1Grouped term ‘musculoskeletal pain/changes’ includes the preferred terms arthralgia, arthritis, back pain, muscle injury, muscle spasms, muscular weakness, musculoskeletal chest pain, myalgia, neck pain, and pain in extremity. 2Grouped term ‘insomnia/sleep disorder’ includes preferred terms insomnia and sleep disorder. 3Grouped term ‘skin reactions’ includes preferred termsdermatitis bullous, dermatitis psoriasiform, dry skin, eczema asteatotic, erythema, rash, skin exfoliation, skin lesion and skin toxicity. 4Grouped term ‘dyspnea’ includes preferred terms dyspnea and dyspnea exertional. 5Grouped term ‘mood disorders’ includes preferred terms affective disorder, agitation, anxiety, depressed mood, depression, irritability, mania and suicide attempt. 6Grouped term ‘cardiac events’ includes preferred terms acute coronary syndrome, angina pectoris, atrial fibrillation, chest pain, hypertension, hypotension and palpitations. 7Grouped term ‘abdominal pain’ includes preferred terms abdominal discomfort, abdominal pain, abdominal pain lower and abdominal pain upper. 8Grouped term ‘clinical liver or bilirubin related events’ includes preferred terms ascites, hepatic encephalopathy, jaundice, ocular icterus, esophageal varices hemorrhage and portal vein thrombosis. 9Grouped term ‘edema’ includes preferred terms edema and edema peripheral. 10Grouped term ‘altered mental status’ includes preferred terms disturbance in attention, memory impairment, psychomotor retardation and somnolence. |
|
Serum Bilirubin Elevations in Patients without Cirrhosis
Serum Bilirubin Elevations/Hepatic Decompensation in Patients with Compensated Cirrhosis
Anemia/Decreased Hemoglobin in Patients without Cirrhosis
Anemia/Decreased Hemoglobin in Patients with Compensated Cirrhosis
7. Drug Interactions
7.3 Established and Other Potential Drug Interactions
| Concomitant Drug Class: Drug Name | Effect on Concentration | Clinical Comments |
| ALPHA1-ADRENORECEPTOR ANTAGONIST | ||
| alfuzosin HCl* | ↑ alfuzosin HCl | Contraindicated due to potential for hypotension [see Contraindications (4)]. |
| ANDROGEN RECEPTOR INHIBITOR | ||
| apalutamide* | ↓ ombitasvir ↓ paritaprevir ↓ ritonavir | Contraindicated due to potential loss of therapeutic activity of TECHNIVIE [see Contraindications (4)]. |
| ANGIOTENSIN RECEPTOR BLOCKERS e.g. | ||
| valsartan*, losartan*, candesartan* | ↑ angiotensin receptor blockers | Decrease the dose of the angiotensin receptor blockers and monitor patients for signs and symptoms of hypotension and/or worsening renal function. If such events occur, consider further dose reduction of the angiotensin receptor blocker or switching to an alternative to the angiotensin receptor blocker. |
| ANTI-ANGINAL | ||
| ranolazine* | ↑ ranolazine | Contraindicated due to potential for serious and/or life-threatening reactions [see Contraindications (4)]. |
| ANTIARRHYTHMICS | ||
| dronedarone* | ↑ dronedarone | Contraindicated due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias [see Contraindications (4)]. |
| digoxin | ↑ digoxin | For contraindicated antiarrhythmics [see Contraindications (4)]. Decrease digoxin dose by 30-50%. Appropriate monitoring of serum digoxin levels is recommended. |
| amiodarone*, bepridil*, disopyramide*, flecainide*, lidocaine (systemic)*, mexiletine*, propafenone*, quinidine* | ↑ antiarrhythmics | Therapeutic monitoring (if available) is recommended for antiarrhythmics when co-administered with TECHNIVIE. |
| ANTICANCER AGENTS/KINASE INHIBITORS | ||
| encorafenib* fostamatinib* ibrutinib* ivosidenib* | ↑ anticancer agents/kinase inhibitors | Co-administration of TECHNIVIE with these anticancer agents/kinase inhibitors may result in increased risk for adverse events. Refer to the prescribing information of these agents for details on co-administration with strong CYP3A inhibitors. |
| ANTICONVULSANTS | ||
| carbamazepine*, phenytoin*, phenobarbital* | ↓ ombitasvir ↓ paritaprevir ↓ ritonavir | Contraindicated due to potential loss of therapeutic activity of TECHNIVIE [see Contraindications (4)]. |
| ANTIDIABETIC DRUGS | ||
| metformin | ↔ metformin | Monitor for signs of onset of lactic acidosis such as respiratory distress, somnolence, and non-specific abdominal distress or worsening renal function. Concomitant metformin use in patients with renal insufficiency or hepatic impairment is not recommended. Refer to the prescribing information of metformin for further guidance. |
| ANTI-GOUT | ||
| colchicine* | ↑ colchicine | Contraindicated due to potential for serious and/or life-threatening reactions in patients with renal and/or hepatic impairment [see Contraindications (4)]. |
| ANTIFUNGALS | ||
| ketoconazole | ↑ ketoconazole | When TECHNIVIE is co-administered with ketoconazole, the maximum daily dose of ketoconazole should be limited to 200 mg per day. |
| voriconazole* | ↓ voriconazole | Co-administration of TECHNIVIE with voriconazole is not recommended unless an assessment of the benefit-to-risk ratio justifies the use of voriconazole. |
| ANTIMYCOBACTERIAL | ||
| rifampin* | ↓ ombitasvir ↓ paritaprevir ↓ ritonavir | Contraindicated due to potential loss of therapeutic activity of TECHNIVIE [see Contraindications (4)]. |
| ANTIPSYCHOTICS | ||
| lurasidone* | ↑ lurasidone | Contraindicated due to potential for serious and/or life-threatening reactions [see Contraindications (4)]. |
| pimozide* | ↑ pimozide | Contraindicated due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias [see Contraindications (4)]. |
| quetiapine* | ↑ quetiapine | For contraindicated antipsychotics [see Contraindications (4)].
|
| CALCIUM CHANNEL BLOCKERS | ||
| amlodipine, nifedipine*, diltiazem*, verapamil* | ↑ calcium channel blockers | Decrease the dose of the calcium channel blocker. The dose of amlodipine should be decreased by at least 50%. Clinical monitoring of patients is recommended for edema and/or signs and symptoms of hypotension. If such events occur, consider further dose reduction of the calcium channel blocker or switching to an alternative to the calcium channel blocker. |
| CORTICOSTEROIDS (INHALED/NASAL) | ||
| fluticasone* | ↑ fluticasone | Concomitant use of TECHNIVIE with inhaled or nasal fluticasone may reduce serum cortisol concentrations. Alternative corticosteroids should be considered, particularly for long term use. |
| DIURETICS | ||
| furosemide | ↑ furosemide (Cmax) | Clinical monitoring of patients is recommended and therapy should be individualized based on patient’s response. |
| ERGOT DERIVATIVES | ||
| ergotamine*, dihydroergotamine*, methylergonovine* | ↑ ergot derivatives | Contraindicated due to potential for acute ergot toxicity characterized by vasospasm. Tissue ischemia has been associated with co-administration of ritonavir and ergonovine, ergotamine, dihydroergotamine, or methylergonovine [see Contraindications (4)]. |
| ETHINYL ESTRADIOL-CONTAINING PRODUCTS | ||
| ethinyl estradiol- containing medications such as combined oral contraceptives | ↔ ethinyl estradiol | Contraindicated due to potential for ALT elevations [see Contraindications (4) and Warnings and Precautions (5.3)]. |
| GI MOTILITY AGENT | ||
| cisapride* | ↑ cisapride | Contraindicated due to potential for serious and/or life threatening reactions such as cardiac arrhythmias [see Contraindications (4)]. |
| GnRH RECEPTOR ANTAGONISTS | ||
| elagolix* | ↑ elagolix | Co-administration of TECHNIVIE with elagolix 200 mg twice daily for more than 1 month is not recommended. |
| HERBAL PRODUCT | ||
| St. John’s Wort* (Hypericum perforatum) | ↓ ombitasvir ↓ paritaprevir ↓ ritonavir | Contraindicated due to potential loss of therapeutic activity of TECHNIVIE [see Contraindications (4)]. |
| HIV-ANTIVIRAL AGENTS | ||
| efavirenz* | ↑ efavirenz ↓ paritaprevir ↓ ritonavir | Contraindicated as co-administration of efavirenz based regimens with paritaprevir and ritonavir was poorly tolerated and resulted in liver enzyme elevations [see Contraindications (4)]. |
| atazanavir or atazanavir/ritonavir | ↑ paritaprevir | Co-administration of TECHNIVIE with atazanavir or atazanavir/ritonavir is not recommended. |
| darunavir/ritonavir | ↓ darunavir (Ctrough) | Treatment naïve patients or treatment experienced patients with no darunavir-associated mutations: Darunavir 800 mg once daily (without ritonavir) can be co-administered with TECHNIVIE. |
| lopinavir/ritonavir | ↑ paritaprevir | Co-administration of TECHNIVIE with lopinavir/ritonavir is not recommended. |
| rilpivirine | ↑ rilpivirine | For contraindicated non-nucleoside reverse transcriptase inhibitors [see Contraindications (4)]. Co-administration of TECHNIVIE with rilpivirine once daily is not recommended due to potential for QT interval prolongation with higher concentrations of rilpivirine. |
| HMG CoA REDUCTASE INHIBITORS | ||
| atorvastatin lovastatin, simvastatin | ↑ atorvastatin ↑ lovastatin, ↑ simvastatin | Contraindicated due to potential for myopathy including rhabdomyolysis [see Contraindications (4)]. |
| pravastatin | ↑ pravastatin | For contraindicated HMG CoA Reductase Inhibitors [see Contraindications (4)]. When TECHNIVIE is co-administered with pravastatin, the dose of pravastatin should not exceed 40 mg per day. |
| IMMUNOSUPPRESSANTS | ||
| everolimus sirolimus tacrolimus | ↑ everolimus ↑ sirolimus ↑ tacrolimus | Contraindicated due to potential for serious and/or life threatening immunosuppressant-associated adverse events [see Contraindications (4)]. |
| cyclosporine | ↑ cyclosporine | For contraindicated immunosuppressants [see Contraindications (4)]. When initiating therapy with TECHNIVIE, reduce cyclosporine dose to 1/5th of the patient’s current cyclosporine dose. Measure cyclosporine blood concentrations to determine subsequent dose modifications. Upon completion of TECHNIVIE therapy, the appropriate time to resume pre-TECHNIVIE dose of cyclosporine should be guided by assessment of cyclosporine blood concentrations. Frequent assessment of renal function and cyclosporine-related side effects is recommended. |
| LONG ACTING BETA-ADRENOCEPTOR AGONIST | ||
| salmeterol* | ↑ salmeterol | Concurrent administration of TECHNIVIE and salmeterol is not recommended. The combination may result in increased risk of cardiovascular adverse events associated with salmeterol, including QT prolongation, palpitations and sinus tachycardia. |
| MUSCLE RELAXANTS | ||
| carisoprodol | ↓ carisoprodol ↔ mepobramate (metabolite of carisoprodol) | Increase dose if clinically indicated. |
| cyclobenzaprine | ↓cyclobenzaprine ↓norcyclobenzaprine (metabolite of cyclobenzaprine) | Increase dose if clinically indicated. |
| MICROSOMAL TRIGLYCERIDE TRANSFER PROTEIN INHIBITOR | ||
| lomitapide* | ↑ lomitapide | Contraindicated due to potential for serious adverse events including hepatotoxicity [see Contraindications (4)]. |
| NARCOTIC ANALGESICS | ||
| buprenorphine/naloxone | ↑ buprenorphine ↑ norbuprenorphine (metabolite of buprenorphine) | Patients should be closely monitored for sedation and cognitive effects. |
| hydrocodone/ acetaminophen fentanyl | ↑ hydrocodone ↔ acetaminophen ↑ fentanyl | Reduce the dose of hydrocodone by 50% and monitor patients for respiratory depression and sedation at frequent intervals. Upon completion of TECHNIVIE therapy, adjust the hydrocodone dose and monitor for signs of opioid withdrawal. Careful monitoring of therapeutic effects and adverse effects of fentanyl (including potentially fatal respiratory depression) is recommended when fentanyl is co-administered with TECHNIVIE. |
| PROTON PUMP INHIBITORS | ||
| omeprazole | ↓ omeprazole | Monitor patients for decreased efficacy of omeprazole. Consider increasing the omeprazole dose in patients whose symptoms are not well controlled; avoid use of more than 40 mg per day of omeprazole. |
| PHOSPHODIESTERASE-5 (PDE5) INHIBITOR | ||
| Sildenafil* when dosed as Revatio for the treatment of pulmonary arterial hypertension (PAH) | ↑ sildenafil | Contraindicated due to potential for sildenafil-associated adverse events such as visual disturbances, hypotension, priapism, and syncope [see Contraindications (4)]. |
| SEDATIVES/HYPNOTICS | ||
| triazolam* orally administered midazolam* | ↑ triazolam ↑ midazolam | Contraindicated due to potential for serious and/or life threatening events such as prolonged or increased sedation or respiratory depression [see Contraindications (4)]. |
| alprazolam | ↑ alprazolam | For contraindicated Sedatives/Hypnotics [see Contraindications (4)]. Clinical monitoring of patients is recommended. A decrease in alprazolam dose can be considered based on clinical response. |
| diazepam | ↓ diazepam ↓ nordiazepam (metabolite of diazepam) | Increase dose if clinically indicated. |
| *Not studied. See Clinical Pharmacology, Tables 7 and 8. The direction of the arrow indicates the direction of the change in exposures (Cmax and AUC) (↑ = increase of more than 20%, ↓ = decrease of more than 20%). |
||
8. Use In Specific Populations
12. Technivie - Clinical Pharmacology
12.3 Pharmacokinetics
| Ombitasvir | Paritaprevir | Ritonavir | |
| Absorption | |||
| Tmax (hr) | ~ 5 | ~ 4-5 | ~ 4-5 |
| Absolute bioavailability (%) | 48 | 53 | NA |
| Effect of moderate fat meal (relative to fasting)a | 1.82 (1.61-2.05) | 3.11 (2.16-4.46) | 1.49 (1.23-1.79) |
| Effect of high fat meal (relative to fasting)a | 1.76 (1.56-1.99) | 2.80 (1.95-4.02) | 1.44 (1.19-1.73) |
| Accumulationb | 0.90- to 1.03-fold | 1.5- to 2-fold | |
| Distribution | |||
| % Bound to human plasma proteins | 99.9 | 97-98.6 | >99 |
| Blood-to-plasma ratio | 0.49 | 0.7 | 0.6 |
| Volume of distribution at steady state (Vss) (L) | 173 | 103 | 21.5c |
| Metabolism | |||
| Metabolism | amide hydrolysis followed by oxidative metabolism | CYP3A4 (major), CYP3A5 | CYP3A (major), CYP2D6 |
| Eliminationd | |||
| Major route of elimination | biliary excretion | metabolism | metabolism |
| t1/2 (hr)e | 21-25 | 5.5 | 4 |
| % of dose excreted in fecesf | 90.2 | 88 | 86.4 |
| % of dose excreted unchanged in fecesf | 87.8 | 1.1 | 33.8 |
| % of dose excreted in urinef | 1.91 | 8.8 | 11.3 |
| % of dose excreted unchanged in urinef | 0.03 | 0.05 | 3.5 |
| NA - data not available a. Values refer to mean non-fasting/fasting ratios (90% Confidence Interval) in systemic exposure (AUC). Moderate fat meal ~600 Kcal, 20-30% calories from fat. High fat meal ~900 Kcal, 60% calories from fat. b. Steady state exposures are achieved after approximately 12 days of dosing. c. It is apparent volume of distribution (V/F) for ritonavir. d. Ombitasvir, paritaprevir, and ritonavir do not inhibit organic anion transporter (OAT1) in vivo and based on in vitro data, are not expected to inhibit organic cation transporter (OCT2), organic anion transporter (OAT3), or multidrug and toxin extrusion proteins (MATE1 and MATE2K) at clinically relevant concentrations. e. t1/2 values refer to the mean elimination half-life. f. Dosing in mass balance studies: single dose administration of [14C]ombitasvir; single dose administration of [14C]paritaprevir co-dosed with 100 mg ritonavir. |
|||
| Pharmacokinetic Parametera | Ombitasvir | Paritaprevir | Ritonavir |
| Cmax (ng/mL) | 82 | 194 | 543 |
| AUC0-24 (ng*h/mL) | 1239 | 2276 | 6072 |
| a. Median values reported based on the population PK analysis. | |||
No dosage adjustment is recommended based on sex or body weight.
No dosage adjustment is recommended based on race or ethnicity.
No dosage adjustment is recommended in geriatric patients [see Use in Specific Populations (8.5)].
See also Contraindications (4), Warnings and Precautions (5.5), Drug Interactions (7)
| Co- administered Drug | Dose of Co-
administered Drug (mg) | n | DAA | Ratio (with/without co-administered drug) of DAA Pharmacokinetic Parameters (90% CI); No Effect = 1.00 |
||
| Cmax | AUC | Cmin | ||||
| Alprazolama | 0.5 single dose | 12 | ombitasvir | 0.98 (0.93, 1.04) | 1.00 (0.96, 1.04) | 0.98 (0.93, 1.04) |
| paritaprevir | 0.91 (0.64, 1.31) | 0.96 (0.73, 1.27) | 1.12 (1.02, 1.23) |
|||
| ritonavir | 0.92 (0.84, 1.02) | 0.96 (0.89, 1.03) | 1.01 (0.94, 1.09) |
|||
| Amlodipinea | 5 single dose | 14 | ombitasvir | 1.00 (0.95, 1.06) | 1.00 (0.97, 1.04) | 1.00 (0.97, 1.04) |
| paritaprevir | 0.77 (0.64, 0.94) | 0.78 (0.68, 0.88) | 0.88 (0.80, 0.95) |
|||
| ritonavir | 0.96 (0.87, 1.06) | 0.93 (0.89, 0.98) | 0.95 (0.89, 1.01) |
|||
| Atazanavirb | 300 once daily | 10 | ombitasvir | 0.83 (0.74, 0.94) | 0.91 (0.81, 1.02) | 0.98 (0.87, 1.11) |
| paritaprevir | 2.74 (1.76, 4.27) | 2.87 (2.08, 3.97) | 3.71 (2.87, 4.79) |
|||
| ritonavir | 0.85 (0.72, 0.99) | 0.97 (0.84, 1.13) | 1.45 (1.29, 1.64) |
|||
| Carbamazepinea | 200 once daily followed by 200 twice daily | 12 | ombitasvir | 0.69 (0.61, 0.78) | 0.69 (0.64, 0.74) | NA |
| paritaprevir | 0.34 (0.25, 0.48) | 0.30 (0.23, 0.38) | NA | |||
| ritonavir | 0.17 (0.12, 0.24) | 0.13 (0.09, 0.17) | NA | |||
| Carisoprodola | 250 single dose | 14 | ombitasvir | 0.98 (0.92, 1.04) | 0.95 (0.92, 0.97) | 0.96 (0.92, 0.99) |
| paritaprevir | 0.88 (0.75, 1.03) | 0.96 (0.85, 1.08) | 1.14 (1.02, 1.27) |
|||
| ritonavir | 0.94 (0.87, 1.02) | 0.94 (0.88, 0.99) | 0.95 (0.89, 1.03) |
|||
| Cyclobenzaprinea | 5 single dose | 14 | ombitasvir | 0.98 (0.92, 1.04) | 1.00 (0.97, 1.03) | 1.01 (0.98, 1.04) |
| paritaprevir | 1.14 (0.99, 1.32) | 1.13 (1.00, 1.28) | 1.13 (1.01, 1.25) |
|||
| ritonavir | 0.93 (0.87, 0.99) | 1.00 (0.95, 1.06) | 1.13 (1.05, 1.21) |
|||
| Cyclosporine | 10 single dosec | 12 | ombitasvir | 1.06 (1.02, 1.11) | 1.10 (1.07, 1.12) | 1.10 (1.06, 1.14) |
| paritaprevir | 1.39 (1.10, 1.75) | 1.46 (1.29, 1.64) | 1.18 (1.08, 1.30) |
|||
| ritonavir | 1.13 (0.94, 1.35) | 1.20 (1.10, 1.30) | 1.11 (0.89, 1.37) |
|||
| Darunavirb | 800 once daily | 9 | ombitasvir | 1.01 (0.87, 1.17) | 1.01 (0.91, 1.11) | 1.06 (0.99, 1.13) |
| paritaprevir | 2.09 (1.35, 3.24) | 1.94 (1.36, 2.75) | 1.85 (1.41, 2.42) |
|||
| ritonavir | 0.83 (0.68, 1.01) | 0.80 (0.73, 0.87) | 0.91 (0.78, 1.06) |
|||
| Diazepama | 2 single dose | 13 | ombitasvir | 1.00 (0.93, 1.08) | 0.98 (0.93, 1.03) | 0.93 (0.88, 0.98) |
| paritaprevir | 0.95 (0.77, 1.18) | 0.91 (0.78, 1.07) | 0.92 (0.82, 1.03) |
|||
| ritonavir | 1.10 (1.02, 1.19) | 1.06 (0.98, 1.14) | 0.98 (0.92, 1.03) |
|||
| Digoxin | 0.5 single dose | 11 | ombitasvir | 0.99 (0.95-1.04) | 1.02 (0.98-1.06) | 1.01 (0.98-1.05) |
| paritaprevir | 1.15 (0.97-1.36) | 1.12 (1.00-1.25) | 0.97 (0.84-1.13) |
|||
| ritonavir | 1.06 (0.99-1.13) | 1.01 (0.98-1.05) | 0.95 (0.86-1.04) |
|||
| Ethinyl estradiol/ Norgestimate | Ethinyl estradiol 0.035 and Norgestimate 0.25 once daily | 7d | ombitasvir | 1.05 (0.81, 1.35) | 0.97 (0.81, 1.15) | 0.96 (0.88, 1.12) |
| paritaprevir | 0.70 (0.40, 1.21) | 0.66 (0.42, 1.04) | 0.87 (0.67, 1.14) |
|||
| ritonavir | 0.80 (0.53, 1.21) | 0.71 (0.54, 0.94) | 0.79 (0.68, 0.93) |
|||
| Everolimusa | 0.75 single dose | 12 | ombitasvir | 0.99 (0.95, 1.03) | 1.02 (0.99, 1.05) | 1.02 (0.99, 1.06) |
| paritaprevir | 1.22 (1.03, 1.43) | 1.26 (1.07, 1.49) | 1.06 (0.97, 1.16) |
|||
| ritonavir | 1.07 (0.99, 1.16) | 1.05 (1.00, 1.10) | 1.07 (1.02, 1.13) |
|||
| Furosemidea | 20 single dose | 12 | ombitasvir | 1.14 (1.03, 1.26) | 1.07 (1.01, 1.12) | 1.12 (1.08, 1.16) |
| paritaprevir | 0.93 (0.63, 1.36) | 0.92 (0.70, 1.21) | 1.26 (1.16, 1.38) |
|||
| ritonavir | 1.10 (0.96, 1.27) | 1.04 (0.92, 1.18) | 1.07 (0.99, 1.17) |
|||
| Hydrocodone/ Acetaminophena | 5/300 single dose | 15 | ombitasvir | 1.01 (0.93, 1.10) | 0.97 (0.93, 1.02) | 0.93 (0.90, 0.97) |
| paritaprevir | 1.01 (0.80, 1.27) | 1.03 (0.89, 1.18) | 1.10 (0.97, 1.26) |
|||
| ritonavir | 1.01 (0.90, 1.13) | 1.03 (0.96, 1.09) | 1.01 (0.93, 1.10) |
|||
| Ketoconazole | 400 once daily | 12 | ombitasvir | 0.98 (0.92, 1.04) | 1.26 (1.20, 1.32) | NA |
| paritaprevir | 1.72 (1.32, 2.26) | 2.16 (1.76, 2.66) | NA | |||
| ritonavir | 1.27 (1.11, 1.45) | 1.51 (1.36, 1.68) | NA | |||
| Lopinavir/ ritonavir | 400/100 twice daily | 18 | ombitasvir | 1.07 (1.01, 1.13) | 1.25 (1.19, 1.32) | 1.48 (1.39, 1.57) |
| paritaprevir | 4.76 (3.54, 6.39) | 6.10 (4.30, 8.67) | 12.33 (7.30, 20.84) |
|||
| ritonavir | 1.74 (1.39, 2.17) | 2.78 (2.42, 3.20) | 10.02 (7.66, 13.11) |
|||
| Lopinavir/ ritonavire | 800/200 once daily | 11 | ombitasvir | 0.97 (0.87, 1.08) | 1.09 (1.00, 1.19) | 1.24 (1.13, 1.35) |
| paritaprevir | 1.78 (1.26, 2.52) | 3.55 (2.37, 5.32) | 14.78 (9.41, 23.23) |
|||
| ritonavir | 1.80 (1.30, 2.48) | 3.09 (2.36, 4.06) | 23.16 (15.55, 34.51) |
|||
| Omeprazole | 40 once daily | 12 | ombitasvir | 0.96 (0.81, 1.14) | 1.00 (0.88, 1.12) | 0.97 (0.89, 1.107) |
| paritaprevir | 1.02 (0.64, 1.62) | 0.93 (0.64, 1.34) | 0.83 (0.67, 1.04) |
|||
| ritonavir | 1.06 (0.95, 1.18) | 1.07 (0.96, 1.21) | 1.07 (0.97, 1.18) |
|||
| Pravastatin | 10 once daily | 10 | ombitasvir | 0.98 (0.90, 1.06) | 0.94 (0.88, 1.02) | 0.97 (0.90, 1.03) |
| paritaprevir | 1.44 (1.15, 1.81) | 1.33 (1.09, 1.62) | 1.28 (0.83, 1.96) |
|||
| ritonavir | 1.37 (1.05, 1.79) | 1.37 (0.84, 2.24) | 0.85 (0.76, 0.96) |
|||
| Rilpivirinea | 25 once daily (morning)f | 10 | ombitasvir | 1.11 (1.02, 1.20) | 1.09 (1.04, 1.14) | 1.05 (1.01, 1.08) |
| paritaprevir | 1.30 (0.94, 1.81) | 1.23 (0.93, 1.64) | 0.95 (0.84, 1.07) |
|||
| ritonavir | 1.10 (0.98, 1.24) | 1.08 (0.93, 1.27) | 0.97 (0.91, 1.04) |
|||
| Sirolimusa | 0.5 single doseh | 11 | ombitasvir | 1.03 (0.93, 1.15) | 1.02 (0.96, 1.09) | 1.05 (0.98, 1.12) |
| paritaprevir | 1.18 (0.91, 1.54) | 1.19 (0.97, 1.46) | 1.16 (1.00, 1.34) |
|||
| ritonavir | 1.00 (0.85, 1.17) | 1.04 (0.94, 1.15) | 1.10 (1.04, 1.17) |
|||
| Tacrolimus | 0.5 single doseg | 11 | ombitasvir | 0.94 (0.89, 1.00) | 0.95 (0.91, 1.00) | 0.95 (0.92, 0.99) |
| paritaprevir | 0.71 (0.55, 0.91) | 0.79 (0.69, 0.92) | 0.84 (0.74, 0.97) |
|||
| ritonavir | 0.884 (0.76, 0.93) | 0.89 (0.85, 0.93) | 1.04 (0.96, 1.13) |
|||
Doses of ombitasvir, paritaprevir, ritonavir were 25 mg, 150 mg and 100 mg, respectively. |
||||||
| Co- administered Drug | Dose of Co- administered Drug (mg) | n | Ratio (with/without TECHNIVIE) of Co-administered Drug Pharmacokinetic Parameters (90% CI); No Effect = 1.00 |
||
| Cmax | AUC | Cmin | |||
| Alprazolama | 0.5 single dose | 12 | 1.09 (1.03, 1.15) | 1.34 (1.15, 1.55) | NA |
| Amlodipinea | 5 single dose | 14 | 1.26 (1.11, 1.44) | 2.57 (2.31, 2.86) | NA |
| Atazanavirb | 300 once daily | 11 | 0.90 (0.83, 0.97) | 0.93 (0.85, 1.02) | 0.81 (0.72, 0.91) |
| Buprenorphine | Buprenorphine: 4 to 24 once daily and Naloxone: 1 to 6 once daily | 11 | 1.19 (1.01, 1.40)c | 1.51 (1.27, 1.78)c | 1.65 (1.30, 2.08)c |
| Norbuprenorphine | 1.82 (1.41, 2.36)c | 2.11 (1.65, 2.70)c | 1.87 (1.48, 2.36)c |
||
| Naloxone | 0.99 (0.84, 1.16)c | 1.11 (0.91, 1.37)c | NA | ||
| Carbamazepinea | 200 once daily followed by 200 twice daily | 12 | 1.10 (1.07, 1.14) | 1.17 (1.13, 1.22) | 1.35 (1.27, 1.45) |
| Carbamazepine’s metabolite, carbamazepine-10,11-epoxide (CBZE) | 0.84 (0.82, 0.87) | 0.75 (0.73, 0.77) | 0.57 (0.54, 0.61) |
||
| Carisoprodola | 250 single dose | 14 | 0.54 (0.47, 0.63) | 0.62 (0.55, 0.70) | NA |
| Carisoprodol's metabolite, meprobamate | 1.17 (1.10, 1.25) | 1.09 (1.03, 1.16) | NA | ||
| Cyclobenzaprinea | 5 single dose | 14 | 0.68 (0.61, 0.75) | 0.60 (0.53, 0.68) | NA |
| Cyclobenzaprine's metabolite norcyclobenzaprine | 1.03 (0.87, 1.23) | 0.74 (0.64, 0.85) | NA | ||
| Cyclosporine | 10 single dosed | 12 | 0.83 (0.72, 0.94)c | 4.28 (3.66, 5.01)c | 12.85 (10.61, 15.55)c |
| Darunavirb | 800 once daily | 9 | 0.99 (0.92, 1.08) | 0.92 (0.84, 1.00) | 0.74 (0.63, 0.88) |
| Diazepama | 2 single dose | 13 | 1.18 (1.07, 1.30) | 0.78 (0.73, 0.82) | NA |
| Diazepam's metabolite nordiazepam | 1.10 (1.03, 1.19) | 0.56 (0.45, 0.70) | NA | ||
| Digoxin | 0.5 single dose | 11 | 1.58 (1.43-1.73) | 1.36 (1.21-1.53) | 1.24 (1.07-1.43) |
| Everolimusa | 0.75 single dose | 12 | 4.74(4.29, 5.25) | 27.12(24.5, 30.1) | 16.10 (14.5, 17.9) |
| Ethinyl Estradiole | Ethinyl estradiol 0.035 and Norgestimate 0.25 once daily | 8 | 1.16 (0.90, 1.50) | 1.06 (0.96, 1.17) | 1.12 (0.94, 1.33) |
| Norelgestromine | 9 | 2.01 (1.77, 2.29) | 2.60 (2.30, 2.95) | 3.11 (2.51, 3.85) |
|
| Norgestrele | 9 | 2.26 (1.91, 2.67) | 2.54 (2.09, 3.09) | 2.93 (2.39, 3.57) |
|
| Furosemidea | 20 single dose | 12 | 1.42 (1.17, 1.72) | 1.08 (1.00, 1.17) | NA |
| Hydrocodonea | 5 single dose | 15 | 1.27 (1.14, 1.40) | 1.90 (1.72, 2.10) | NA |
| Ketoconazole | 400 once daily | 12 | 1.10 (1.05, 1.16) | 2.05 (1.93, 2.18) | NA |
| Lopinavir/ritonavirf | 400/100 twice daily | 18 | 1.06 (0.99, 1.14) | 1.13 (1.09, 1.17) | 1.34 (1.26, 1.42) |
| Lopinavir/ritonavirf,g | 800/200 once daily | 12 | 1.05 (0.95, 1.17) | 1.17 (1.09, 1.26) | 3.50 (2.69, 4.56) |
| Omeprazole | 40 once daily | 12 | 0.48 (0.29, 0.78) | 0.46 (0.27, 0.77) | NA |
| Pravastatin | 10 once daily | 10 | 1.43 (1.09, 1.88) | 1.76 (1.46, 2.13) | NA |
| Rilpivirinea | 25 once daily (morning)h | 8 | 2.55 (2.08, 3.12) | 3.25 (2.80, 3.77) | 3.62 (3.12, 4.21) |
| Sirolimusa | 0.5 single dosej | 11 | 6.40 (5.34, 7.68)c | 37.99 (31.5, 45.8)c | 19.55 (16.7, 22.9)c |
| Tacrolimus | 0.5 single dosei | 11 | 4.27 (3.49, 5.22)c | 85.81 (67.88, 108.49)c | 24.61 (19.69, 30.77)c |
NA: not available/not applicable; CI: Confidence interval. Doses of ombitasvir, paritaprevir and ritonavir were 25 mg, 150 mg and 100 mg, respectively. |
|||||
13. Nonclinical Toxicology
14. Clinical Studies
14.2 Clinical Trial Results in Adults with Chronic GT4 HCV Infection without Cirrhosis
| Treatment outcome | Ombitasvir + Paritaprevir
+ Ritonavir with RBV for 12 weeks | Ombitasvir + Paritaprevir
+ Ritonavir for 12 weeks |
|
| Treatment-naïve | Treatment-experienced | Treatment-naïve | |
| % (n/N) | % (n/N) | % (n/N) | |
| Overall SVR12 | 100% (42/42) | 100% (49/49) | 91% (40/44) |
| Outcome for subjects without SVR12 | |||
| On-treatment VFa | 0% (0/42) | 0% (0/49) | 2% (1/44) |
| Relapseb | 0% (0/42) | 0% (0/49) | 5% (2/42) |
| Otherc | 0% (0/42) | 0% (0/49) | 2% (1/44) |
VF = virologic failure
|
|||
14.3 Clinical Trial Results in Adults with Chronic GT4 HCV Infection with Compensated Cirrhosis
| Treatment outcome | TECHNIVIE with RBV for 12 Weeks |
| % (n/N) | |
| SVR12, % (n/N) | 97% (57/59) |
| Outcome for subjects without SVR12 | |
| On-treatment VFa | 2% (1/59) |
| Relapseb | 0 (0/57) |
| Otherc | 2% (1/59) |
|
|
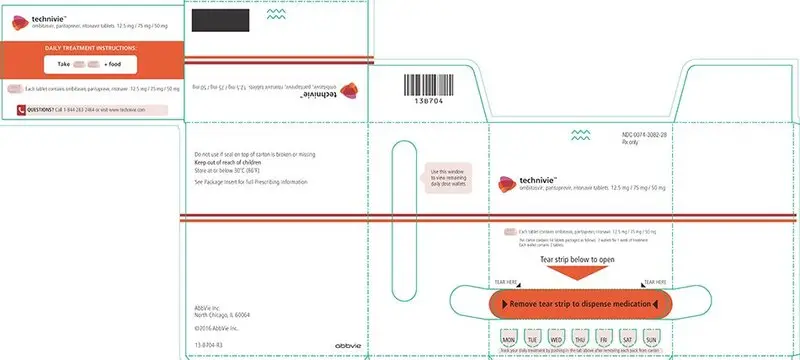
16. How is Technivie supplied
Each child resistant daily dose pack contains two TECHNIVIE tablets. The NDC number is 0074-3082-28.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Inform patients to review the Medication Guide for ribavirin [see Warnings and Precautions (5.3)].
Risk of Hepatitis B Virus Reactivation in Patients Coinfected with HCV and HBV
Risk of ALT Elevations or Hepatic Decompensation and Failure
Advise patients not to remove tablets from the daily dose pack until they are ready to take them.
Manufactured by AbbVie Inc., North Chicago, IL 60064.
TECHNIVIE and NORVIR are trademarks of AbbVie Inc.
© 2019 AbbVie Inc. All rights reserved.
03-C074
| MEDICATION GUIDE TECHNIVIE (TEK-ni-vee) (ombitasvir, paritaprevir and ritonavir tablets) |
|
| Important: TECHNIVIE is taken in combination with ribavirin. You should also read the Medication Guide that comes with ribavirin. | |
| What is the most important information I should know about TECHNIVIE?
TECHNIVIE can cause serious side effects, including:
|
|
|
|
| For more information about side effects, see the section “What are the possible side effects of TECHNIVIE?” | |
| What is TECHNIVIE?
TECHNIVIE is a prescription medicine used with ribavirin to treat adults with genotype 4 chronic (lasting a long time) hepatitis C virus (HCV) infection without cirrhosis or with a certain type of cirrhosis (compensated). You should also read the Medication Guide for ribavirin. TECHNIVIE can be used in people who have compensated cirrhosis. TECHNIVIE is not for people with advanced cirrhosis (decompensated). If you have cirrhosis, talk to your healthcare provider before taking TECHNIVIE. Each TECHNIVIE tablet contains the medicines ombitasvir, paritaprevir and ritonavir. It is not known if TECHNIVIE is safe and effective in children under 18 years of age. |
|
Do not take TECHNIVIE if you:
|
|
Before taking TECHNIVIE, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines interact with TECHNIVIE. Keep a list of your medicines to show your healthcare provider and pharmacist.
|
|
|
|
|
What are the possible side effects of TECHNIVIE? TECHNIVIE can cause serious side effects, including: See “What is the most important information I should know about TECHNIVIE?” Common side effects of TECHNIVIE when used with ribavirin include: |
|
|
|
| These are not all the possible side effects of TECHNIVIE. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |
Keep TECHNIVIE and all medicines out of the reach of children. |
|
|
General information about the safe and effective use of TECHNIVIE |
|
|
What are the ingredients in TECHNIVIE? Active ingredients: ombitasvir, paritaprevir, and ritonavir Manufactured by AbbVie Inc., North Chicago, IL 60064. TECHNIVIE and NORVIR are trademarks of AbbVie Inc. For more information, go to www.technivie.com or call 1-844-283-2464. |
|
| This Medication Guide has been approved by the U.S. Food and Drug Administration. 03-C074 | Revised: December 2019 |
ombitasvir, paritaprevir, ritonavir tablets 12.5 mg/ 75 mg/ 50 mg
Each tablet contains ombitasvir, paritaprevir, ritonavir 12.5 mg/ 75 mg/ 50 mg
This carton contains 14 Tablets packaged as follows: 7 wallets for 1 week of treatment.
Each wallet contains 2 tablets.
Do not use if seal on top of carton is broken or missing
See Package Insert for full Prescribing Information
ombitasvir, paritaprevir, ritonavir tablets 12.5 mg/ 75 mg/ 50 mg
Each tablet contains ombitasvir, paritaprevir, ritonavir 12.5 mg/ 75 mg/ 50 mg
| TECHNIVIE
ombitasvir and paritaprevir and ritonavir kit |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - AbbVie Inc. (078458370) |