Drug Detail:Toradol (Ketorolac (oral/injection) [ kee-toe-role-ak ])
Drug Class: Nonsteroidal anti-inflammatory drugs
WARNING
TORADOLORAL (ketorolac tromethamine), a nonsteroidal anti-inflammatory drug (NSAID), is indicated for the short-term (up to 5 days in adults), management of moderately severe acute pain that requires analgesia at the opioid level and only as continuation treatment following IV or IM dosing of ketorolac tromethamine, if necessary. The total combined duration of use of TORADOLORAL and ketorolac tromethamine should not exceed 5 days.
TORADOLORAL is not indicated for use in pediatric patients and it is NOT indicated for minor or chronic painful conditions. Increasing the dose of TORADOLORAL beyond a daily maximum of 40 mg in adults will not provide better efficacy but will increase the risk of developing serious adverse events.
Toradol - Clinical Pharmacology
Kinetics in Special Populations
Race
Pharmacokinetic differences due to race have not been identified.
| Pharmacokinetic Parameters (units) | Oral* | Intramuscular† | Intravenous Bolus‡ | |||
|---|---|---|---|---|---|---|
| 10 mg | 15 mg | 30 mg | 60 mg | 15 mg | 30 mg | |
| % Dose metabolized = <50 | ||||||
| % Dose excreted in feces = 6 | ||||||
| % Dose excreted in urine = 91 | ||||||
| % Plasma protein binding = 99 | ||||||
|
||||||
| Bioavailability (extent) | 100% | |||||
| Tmax§ (min) | 44 ± 34 | 33 ± 21¶ | 44 ± 29 | 33 ± 21¶ | 1.1 ± 0.7¶ | 2.9 ± 1.8 |
| Cmax# (µg/mL) [single-dose] | 0.87 ± 0.22 | 1.14 ± 0.32¶ | 2.42 ± 0.68 | 4.55 ± 1.27¶ | 2.47 ± 0.51¶ | 4.65 ± 0.96 |
| Cmax (µg/mL) [steady state qid] | 1.05 ± 0.26¶ | 1.56 ± 0.44¶ | 3.11 ± 0.87¶ | N/AÞ | 3.09 ± 1.17¶ | 6.85 ± 2.61 |
| Cminß (µg/mL) [steady state qid] | 0.29 ± 0.07¶ | 0.47 ± 0.13¶ | 0.93 ± 0.26¶ | N/A | 0.61 ± 0.21¶ | 1.04 ± 0.35 |
| Cavgà (µg/mL) [steady state qid] | 0.59 ± 0.20¶ | 0.94 ± 0.29¶ | 1.88 ± 0.59¶ | N/A | 1.09 ± 0.30¶ | 2.17 ± 0.59 |
| Vβè (L/kg) | ————— 0.175 ± 0.039 ———— | 0.210 ± 0.044 | ||||
| Total Clearance [in L/h/kg]‡ | Terminal Half-life [in hours] | |||
|---|---|---|---|---|
| Type of Subjects | IM | ORAL | IM | ORAL |
| Mean (range) | Mean (range) | Mean (range) | Mean (range) | |
|
||||
| Normal Subjects IM (n=54) mean age=32, range=18–60 Oral (n=77) mean age=32, range=20–60 | 0.023 (0.010–0.046) | 0.025 (0.013–0.050) | 5.3 (3.5–9.2) | 5.3 (2.4–9.0) |
| Healthy Elderly Subjects IM (n=13), Oral (n=12) mean age=72, range=65–78 | 0.019 (0.013–0.034) | 0.024 (0.018–0.034) | 7.0 (4.7–8.6) | 6.1 (4.3–7.6) |
| Patients with Hepatic Dysfunction IM and Oral (n=7) mean age=51, range=43–64 | 0.029 (0.013–0.066) | 0.033 (0.019–0.051) | 5.4 (2.2–6.9) | 4.5 (1.6–7.6) |
| Patients with Renal Impairment IM (n=25), Oral (n=9) serum creatinine=1.9–5.0 mg/dL, mean age (IM)=54, range=35–71 mean age (Oral)=57, range=39–70 | 0.015 (0.005–0.043) | 0.016 (0.007–0.052) | 10.3 (5.9–19.2) | 10.8 (3.4–18.9) |
| Renal Dialysis Patients IM and Oral (n=9) mean age=40, range=27–63 | 0.016 (0.003–0.036) | — | 13.6 (8.0–39.1) | — |
Related/similar drugs
aspirin, acetaminophen, tramadol, naproxen, oxycodone, Tylenol, fentanylClinical Studies
Contraindications
(see also Boxed WARNING)
TORADOL is contraindicated in patients with previously demonstrated hypersensitivity to ketorolac tromethamine.
TORADOL is contraindicated in patients with active peptic ulcer disease, in patients with recent gastrointestinal bleeding or perforation and in patients with a history of peptic ulcer disease or gastrointestinal bleeding.
TORADOL should not be given to patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients (see WARNINGS: Anaphylactoid Reactions, and PRECAUTIONS: Preexisting Asthma).
TORADOL is contraindicated as prophylactic analgesic before any major surgery.
TORADOL is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS).
TORADOL is contraindicated in patients with advanced renal impairment or in patients at risk for renal failure due to volume depletion (see WARNINGS for correction of volume depletion).
TORADOL is contraindicated in labor and delivery because, through its prostaglandin synthesis inhibitory effect, it may adversely affect fetal circulation and inhibit uterine contractions, thus increasing the risk of uterine hemorrhage.
TORADOL inhibits platelet function and is, therefore, contraindicated in patients with suspected or confirmed cerebrovascular bleeding, hemorrhagic diathesis, incomplete hemostasis and those at high risk of bleeding (see WARNINGS and PRECAUTIONS).
TORADOL is contraindicated in patients currently receiving aspirin or NSAIDs because of the cumulative risks of inducing serious NSAID-related adverse events.
The concomitant use of TORADOL and probenecid is contraindicated.
The concomitant use of ketorolac tromethamine and pentoxifylline is contraindicated.
Warnings
(see also Boxed WARNING)
The total combined duration of use of TORADOLORAL and IV or IM dosing of ketorolac tromethamine is not to exceed 5 days in adults. TORADOLORAL is not indicated for use in pediatric patients.
The most serious risks associated with TORADOL are:
Gastrointestinal Effects – Risk of Ulceration, Bleeding, and Perforation
TORADOL is contraindicated in patients with previously documented peptic ulcers and/or GI bleeding. Toradol can cause serious gastrointestinal (GI) adverse events including bleeding, ulceration and perforation, of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with TORADOL.
Only one in five patients who develop a serious upper GI adverse event on NSAID therapy is symptomatic. Minor upper gastrointestinal problems, such as dyspepsia, are common and may also occur at any time during NSAID therapy. The incidence and severity of gastrointestinal complications increases with increasing dose of, and duration of treatment with, TORADOL. Do not use TORADOL for more than five days. However, even short-term therapy is not without risk. In addition to past history of ulcer disease, other factors that increase the risk for GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids, or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore, special care should be taken in treating this population.
To minimize the potential risk for an adverse GI event, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI adverse event is suspected. This should include discontinuation of TORADOL until a serious GI adverse event is ruled out. For high risk patients, alternate therapies that do not involve NSAIDs should be considered.
NSAIDs should be given with care to patients with a history of inflammatory bowel disease (ulcerative colitis, Crohn's disease) as their condition may be exacerbated.
Renal Effects
Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a NSAID may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
TORADOL and its metabolites are eliminated primarily by the kidneys, which, in patients with reduced creatinine clearance, will result in diminished clearance of the drug (see CLINICAL PHARMACOLOGY). Therefore, TORADOL should be used with caution in patients with impaired renal function (see DOSAGE AND ADMINISTRATION) and such patients should be followed closely. With the use of TORADOL, there have been reports of acute renal failure, interstitial nephritis and nephrotic syndrome.
Anaphylactoid Reactions
As with other NSAIDs, anaphylactoid reactions may occur in patients without a known previous exposure or hypersensitivity to TORADOL. TORADOL should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs (see CONTRAINDICATIONS and PRECAUTIONS: Preexisting Asthma). Anaphylactoid reactions, like anaphylaxis, may have a fatal outcome. Emergency help should be sought in cases where an anaphylactoid reaction occurs.
Precautions
General
TORADOL cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids.
The pharmacological activity of TORADOL in reducing inflammation may diminish the utility of this diagnostic sign in detecting complications of presumed noninfectious, painful conditions.
Hepatic Effect
TORADOL should be used with caution in patients with impaired hepatic function or a history of liver disease. Borderline elevations of one or more liver tests may occur in up to 15% of patients taking NSAIDs including TORADOL. These laboratory abnormalities may progress, may remain unchanged, or may be transient with continuing therapy. Notable elevations of ALT or AST (approximately three or more times the upper limit of normal) have been reported in approximately 1% of patients in clinical trials with NSAIDs. In addition, rare cases of severe hepatic reactions, including jaundice and fatal fulminant hepatitis, liver necrosis and hepatic failure, some of them with fatal outcomes have been reported.
A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test has occurred, should be evaluated for evidence of the development of a more severe hepatic reaction while on therapy with TORADOL. If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), TORADOL should be discontinued.
Hematologic Effect
Anemia is sometimes seen in patients receiving NSAIDs, including TORADOL. This may be due to fluid retention, occult or gross GI blood loss, or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs, including TORADOL, should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia. NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible. Patients receiving TORADOL who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants, should be carefully monitored.
Preexisting Asthma
Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm which can be fatal. Since cross reactivity, including bronchospasm, between aspirin and other nonsteroidal anti-inflammatory drugs has been reported in such aspirin-sensitive patients, TORADOL should not be administered to patients with this form of aspirin sensitivity and should be used with caution in patients with preexisting asthma.
Information for Patients
TORADOL is a potent NSAID and may cause serious side effects such as gastrointestinal bleeding or kidney failure, which may result in hospitalization and even fatal outcome.
Physicians, when prescribing TORADOL, should inform their patients or their guardians of the potential risks of TORADOL treatment (see Boxed WARNING, WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS sections), instruct patients to seek medical advice if they develop treatment-related adverse events, and advise patients not to give TORADOLORAL to other family members and to discard any unused drug.
Remember that the total combined duration of use of TORADOLORAL and IV or IM dosing of ketorolac tromethamine is not to exceed 5 days in adults. TORADOLORAL is not indicated for use in pediatric patients.
Patients should be informed of the following information before initiating therapy with an NSAID and periodically during the course of ongoing therapy. Patients should also be encouraged to read the NSAID Medication Guide that accompanies each prescription dispensed.
- TORADOL, like other NSAIDs, may cause serious CV side effects, such as MI or stroke, which may result in hospitalization and even death. Although serious CV events can occur without warning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and should ask for medical advice when observing any indicative sign or symptoms. Patients should be apprised of the importance of this follow-up (see WARNINGS: Cardiovascular Effects).
- TORADOL, like other NSAIDs, can cause GI discomfort and rarely, serious GI side effects, such as ulcers and bleeding, which may result in hospitalization and even death. Although serious GI tract ulcerations and bleeding can occur without warning symptoms, patients should be alert for the signs and symptoms of ulcerations and bleeding, and should ask for medical advice when observing any indicative sign or symptoms including epigastric pain, dyspepsia, melena, and hematemesis. Patients should be apprised of the importance of this follow-up (see WARNINGS: Gastrointestinal Effects – Risk of Ulceration, Bleeding, and Perforation).
- TORADOL, like other NSAIDs, can cause serious skin side effects such as exfoliative dermatitis, SJS, and TEN, which may result in hospitalizations and even death. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching, and should ask for medical advice when observing any indicative signs or symptoms. Patients should be advised to stop the drug immediately if they develop any type of rash and contact their physicians as soon as possible.
- Patients should promptly report signs or symptoms of unexplained weight gain or edema to their physicians.
- Patients should be informed of the warning signs and symptoms of hepatotoxicity (eg, nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness, and "flu-like" symptoms). If these occur, patients should be instructed to stop therapy and seek immediate medical therapy.
- Patients should be informed of the signs of an anaphylactoid reaction (eg, difficulty breathing, swelling of the face or throat). If these occur, patients should be instructed to seek immediate emergency help (see WARNINGS).
- In late pregnancy, as with other NSAIDs, TORADOL should be avoided because it will cause premature closure of the ductus arteriosus.
Drug Interactions
Ketorolac is highly bound to human plasma protein (mean 99.2%). There is no evidence in animal or human studies that TORADOL induces or inhibits hepatic enzymes capable of metabolizing itself or other drugs.
Nursing Mothers
Limited data from one published study involving 10 breastfeeding women 2-6 days postpartum showed low levels of ketorolac in breast milk. Levels were undetectable (less than 5 ng/mL) in 4 of the patients. After a single administration of 10 mg of TORADOLORAL , the maximum milk concentration observed was 7.3 ng/mL, and the maximum milk-to-plasma ratio was 0.037. After 1 day of dosing (10 mg every 6 hours), the maximum milk concentration was 7.9 ng/mL, and the maximum milk-to-plasma ratio was 0.025. Assuming a daily intake of 400-1,000 mL of human milk per day and a maternal body weight of 60 kg, the calculated maximum daily infant exposure was 0.00263 mg/kg/day, which is 0.4% of the maternal weight-adjusted dose.
Exercise caution when ketorolac is administered to a nursing woman. Available information has not shown any specific adverse events in nursing infants; however, instruct patients to contact their infant's health care provider if they note any adverse events.
Pediatric Use
TORADOLORAL is not indicated for use in pediatric patients. The safety and effectiveness of TORADOLORAL in pediatric patients below the age of 17 have not been established.
Geriatric Use (≥65 years of age)
Because ketorolac tromethamine may be cleared more slowly by the elderly (see CLINICAL PHARMACOLOGY) who are also more sensitive to the dose-related adverse effects of NSAIDs (see WARNINGS: Gastrointestinal Effects – Risk of Ulceration, Bleeding, and Perforation), extreme caution, reduced dosages (see DOSAGE AND ADMINISTRATION), and careful clinical monitoring must be used when treating the elderly with TORADOL.
Adverse Reactions/Side Effects
Adverse reaction rates increase with higher doses of TORADOL. Practitioners should be alert for the severe complications of treatment with TORADOL, such as GI ulceration, bleeding and perforation, postoperative bleeding, acute renal failure, anaphylactic and anaphylactoid reactions and liver failure (see Boxed WARNING, WARNINGS, PRECAUTIONS, and DOSAGE AND ADMINISTRATION). These NSAID-related complications can be serious in certain patients for whom TORADOL is indicated, especially when the drug is used inappropriately.
In patients taking TORADOL or other NSAIDs in clinical trials, the most frequently reported adverse experiences in approximately 1% to 10% of patients are:
|
||
| Gastrointestinal (GI) experiences including: | ||
| abdominal pain* | constipation/diarrhea | dyspepsia* |
| flatulence | GI fullness | GI ulcers (gastric/duodenal) |
| gross bleeding/perforation | heartburn | nausea* |
| stomatitis | vomiting | |
| Other experiences: | ||
| abnormal renal function | anemia | dizziness |
| drowsiness | edema | elevated liver enzymes |
| headaches* | hypertension | increased bleeding time |
| injection site pain | pruritus | purpura |
| rashes | tinnitus | sweating |
Additional adverse experiences reported occasionally (<1% in patients taking TORADOL or other NSAIDs in clinical trials) include:
Body as a Whole: fever, infections, sepsis
Cardiovascular: congestive heart failure, palpitation, pallor, tachycardia, syncope
Dermatologic: alopecia, photosensitivity, urticaria
Gastrointestinal: anorexia, dry mouth, eructation, esophagitis, excessive thirst, gastritis, glossitis, hematemesis, hepatitis, increased appetite, jaundice, melena, rectal bleeding
Hemic and Lymphatic: ecchymosis, eosinophilia, epistaxis, leukopenia, thrombocytopenia
Metabolic and Nutritional: weight change
Nervous System: abnormal dreams, abnormal thinking, anxiety, asthenia, confusion, depression, euphoria, extrapyramidal symptoms, hallucinations, hyperkinesis, inability to concentrate, insomnia, nervousness, paresthesia, somnolence, stupor, tremors, vertigo, malaise
Reproductive, female: infertility
Respiratory: asthma, cough, dyspnea, pulmonary edema, rhinitis
Special Senses: abnormal taste, abnormal vision, blurred vision, hearing loss
Urogenital: cystitis, dysuria, hematuria, increased urinary frequency, interstitial nephritis, oliguria/polyuria, proteinuria, renal failure, urinary retention
Other rarely observed reactions (reported from postmarketing experience in patients taking TORADOL or other NSAIDs) are:
Body as a Whole: angioedema, death, hypersensitivity reactions such as anaphylaxis, anaphylactoid reaction, laryngeal edema, tongue edema (see WARNINGS), myalgia
Cardiovascular: arrhythmia, bradycardia, chest pain, flushing, hypotension, myocardial infarction, vasculitis
Dermatologic: exfoliative dermatitis, erythema multiforme, Lyell's syndrome, bullous reactions including Stevens-Johnson syndrome and toxic epidermal necrolysis
Gastrointestinal: acute pancreatitis, liver failure, ulcerative stomatitis, exacerbation of inflammatory bowel disease (ulcerative colitis, Crohn's disease)
Hemic and Lymphatic: agranulocytosis, aplastic anemia, hemolytic anemia, lymphadenopathy, pancytopenia, postoperative wound hemorrhage (rarely requiring blood transfusion — see Boxed WARNING, WARNINGS, and PRECAUTIONS)
Metabolic and Nutritional: hyperglycemia, hyperkalemia, hyponatremia
Nervous System: aseptic meningitis, convulsions, coma, psychosis
Respiratory: bronchospasm, respiratory depression, pneumonia
Special Senses: conjunctivitis
Urogenital: flank pain with or without hematuria and/or azotemia, hemolytic uremic syndrome
Postmarketing Surveillance Study
A large postmarketing observational, nonrandomized study, involving approximately 10,000 patients receiving ketorolac tromethamineIV/IM, demonstrated that the risk of clinically serious gastrointestinal (GI) bleeding was dose-dependent (see Tables 3A and 3B). This was particularly true in elderly patients who received an average daily dose greater than 60 mg/day of ketorolac tromethamineIV/IM (see Table 3A).
| A. Adult Patients Without History of PUB | ||||
| Age of Patients | Total Daily Dose of Ketorolac TromethamineIV/IM | |||
| ≤60 mg | >60 to 90 mg | >90 to 120 mg | >120 mg | |
| <65 years of age | 0.4% | 0.4% | 0.9% | 4.6% |
| ≥65 years of age | 1.2% | 2.8% | 2.2% | 7.7% |
| B. Adult Patients With History of PUB | ||||
| Age of Patients | Total Daily Dose of Ketorolac TromethamineIV/IM | |||
| ≤60 mg | >60 to 90 mg | >90 to 120 mg | >120 mg | |
| <65 years of age | 2.1% | 4.6% | 7.8% | 15.4% |
| ≥65 years of age | 4.7% | 3.7% | 2.8% | 25.0% |
Symptoms and Signs
Symptoms following acute NSAID overdoses are usually limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which are generally reversible with supportive care. Gastrointestinal bleeding can occur. Hypertension, acute renal failure, respiratory depression and coma may occur, but are rare. Anaphylactoid reactions have been reported with therapeutic ingestion of NSAIDs, and may occur following an overdose.
Toradol Dosage and Administration
Carefully consider the potential benefits and risks of TORADOL and other treatment options before deciding to use TORADOL. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals. In adults, the combined duration of use of IV or IM dosing of ketorolac tromethamine and TORADOLORAL is not to exceed 5 days. In adults, the use of TORADOLORAL is only indicated as continuation therapy to IV or IM dosing of ketorolac tromethamine.
Transition from IV or IM dosing of ketorolac tromethamine (single- or multiple-dose) to multiple-dose TORADOLORAL:
Patients age 17 to 64: 20 mg PO once followed by 10 mg q4-6 hours prn not >40 mg/day
Patients age ≥65, renally impaired, and/or weight<50 kg (110 lbs): 10 mg PO once followed by 10 mg q4-6 hours prn not >40 mg/day
Note:
Oral formulation should not be given as an initial dose
Use minimum effective dose for the individual patient
Do not shorten dosing interval of 4 to 6 hours
Total duration of treatment in adult patients: the combined duration of use of IV or IM dosing of ketorolac tromethamine and TORADOLORAL is not to exceed 5 days.
The following table summarizes TORADOLORAL dosing instructions in terms of age group:
| Patient Population | TORADOLORAL
(following IV or IM dosing of ketorolac tromethamine) |
|---|---|
| Age <17 years | Oral not approved |
| Adult Age 17 to 64 years | 20 mg once, then 10 mg q4-6 hours prn not >40 mg/day |
| Adult Age ≥65 years, renally impaired, and/or weight <50 kg | 10 mg once, then 10 mg q4-6 hours prn not >40 mg/day |
How is Toradol supplied
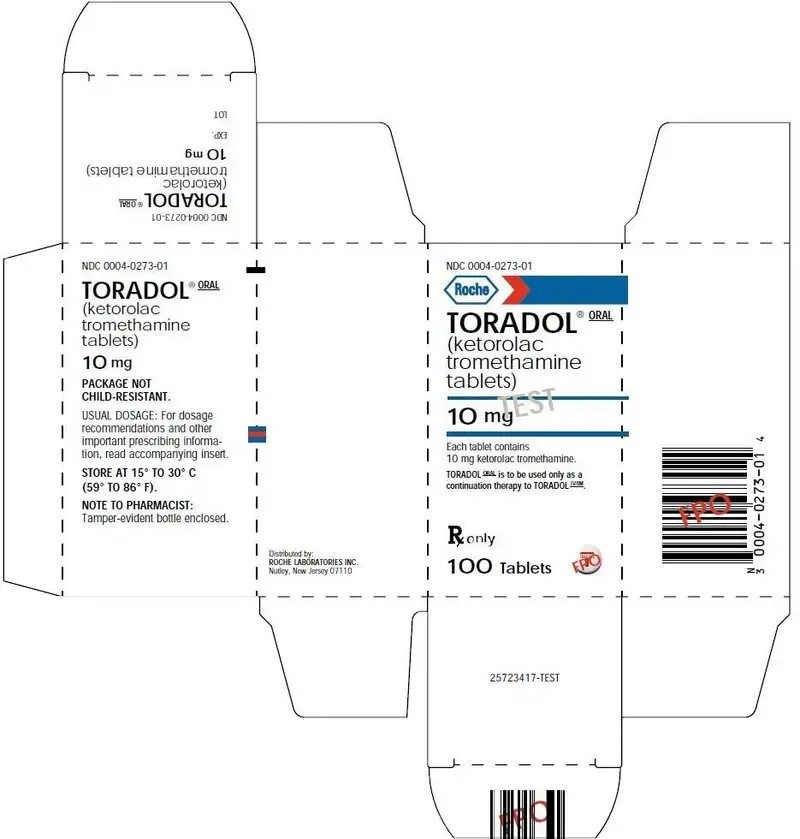
TORADOLORAL 10 mg tablets are round, white, film-coated, red printed tablets. There is a large T printed on both sides of the tablet, with TORADOL on one side, and ROCHE on the other, available in bottles of 100 tablets (NDC 0004-0273-01).
| TORADOL
ketorolac tromethamine tablet, film coated |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Genentech, Inc. (080129000) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Roche Ireland Limited | 219656998 | API MANUFACTURE(0004-0273) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Productos Roche S.A. de C.V. | 588441795 | MANUFACTURE(0004-0273) , PACK(0004-0273) , ANALYSIS(0004-0273) | |