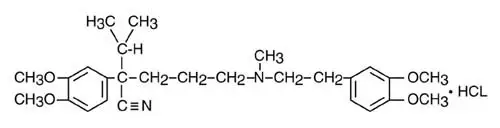
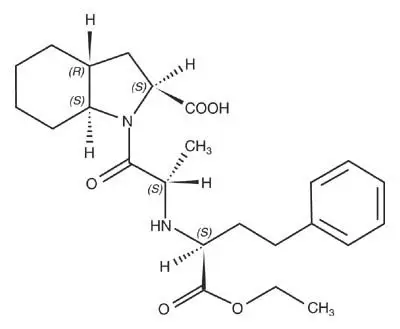
Drug Detail:Trandolapril and verapamil (Trandolapril and verapamil [ tran-dol-a-pril-and-ver-ap-a-mil ])
Drug Class: ACE inhibitors with calcium channel blocking agents
WARNING: FETAL TOXICITY
- When pregnancy is detected, discontinue trandolapril/verapamil hydrochloride ER tablets as soon as possible.
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus(see WARNINGS: Fetal Toxicity).
Related/similar drugs
amlodipine, lisinopril, metoprolol, losartan, furosemide, hydrochlorothiazideTrandolapril and Verapamil - Clinical Pharmacology
Indications and Usage for Trandolapril and Verapamil
Trandolapril/verapamil hydrochloride ER tablets are indicated for the treatment of hypertension.
Contraindications
- Severe left ventricular dysfunction (see WARNINGS).
- Hypotension (systolic pressure less than 90 mmHg) or cardiogenic shock.
- Sick sinus syndrome (except in patients with a functioning artificial ventricular pacemaker).
- Second- or third-degree AV block (except in patients with a functioning artificial ventricular pacemaker).
- Patients with atrial flutter or atrial fibrillation and an accessory bypass tract (e.g. Wolff-Parkinson-White, Lown-Ganong-Levine syndromes) (see WARNINGS).
- Patients taking flibanserin (see PRECAUTIONS, Drug Interactions).
Warnings
Anaphylactoid and Possibly Related Reactions
Patient Counseling Information
Trandolapril Component
Non-Steroidal Anti-Inflammatory Agents including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
Other (Verapamil Component)
Pediatric Use
Neonates with a history of in utero exposure to trandolapril/verapamil hydrochloride ER tablets:
Adverse Reactions/Side Effects
| TRANDOLAPRIL/VERAPAMIL HYDROCHLORIDE ER TABLETS
(N = 541) % Incidence (% Discontinuance) | PLACEBO
(N = 206) % Incidence (% Discontinuance) |
|
| AV Block First Degree | 3.9 (0.2) | 0.5 (0.0) |
| Bradycardia | 1.8 (0.0) | 0.0 (0.0) |
| Bronchitis | 1.5 (0.0) | 0.5 (0.0) |
| Chest Pain | 2.2 (0.0) | 1.0 (0.0) |
| Constipation | 3.3 (0.0) | 1.0 (0.0) |
| Cough | 4.6 (0.0) | 2.4 (0.0) |
| Diarrhea | 1.5 (0.2) | 1.0 (0.0) |
| Dizziness | 3.1 (0.0) | 1.9 (0.5) |
| Dyspnea | 1.3 (0.4) | 0.0 (0.0) |
| Edema | 1.3 (0.0) | 2.4 (0.0) |
| Fatigue | 2.8 (0.4) | 2.4 (0.0) |
| Headache(s)+ | 8.9 (0.0) | 9.7 (0.5) |
| Increased Liver Enzymes* | 2.8 (0.2) | 1.0 (0.0) |
| Nausea | 1.5 (0.2) | 0.5 (0.0) |
| Pain Extremity(ies) | 1.1 (0.2) | 0.5 (0.0) |
| Pain Back+ | 2.2 (0.0) | 2.4 (0.0) |
| Pain Joint(s) | 1.7 (0.0) | 1.0 (0.0) |
| Upper Respiratory Tract Infection(s)+ | 5.4 (0.0) | 7.8 (0.0) |
| Upper Respiratory Tract Congestion+ | 2.4 (0.0) | 3.4 (0.0) |
| * Also includes increase in SGPT, SGOT, Alkaline Phosphatase + Incidence of adverse events is higher in Placebo group than trandolapril/verapamil hydrochloride ER tablets patients |
||
Trandolapril and Verapamil Dosage and Administration
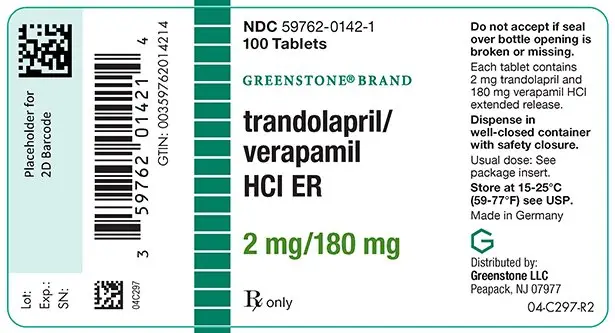
How is Trandolapril and Verapamil supplied
NDC 59762-0142-1 - bottles of 100
NDC 59762-0132-1 - bottles of 100
| TRANDOLAPRIL AND VERAPAMIL HYDROCHLORIDE
ER
trandolapril and verapamil hydrochloride tablet, film coated, extended release |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| TRANDOLAPRIL AND VERAPAMIL HYDROCHLORIDE
ER
trandolapril and verapamil hydrochloride tablet, film coated, extended release |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| TRANDOLAPRIL AND VERAPAMIL HYDROCHLORIDE
ER
trandolapril and verapamil hydrochloride tablet, film coated, extended release |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| TRANDOLAPRIL AND VERAPAMIL HYDROCHLORIDE
ER
trandolapril and verapamil hydrochloride tablet, film coated, extended release |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Greenstone LLC (825560733) |