Drug Detail:Tremfya (Guselkumab [ gue-sel-koo-mab ])
Drug Class: Interleukin inhibitors
Highlights of Prescribing Information
TREMFYA ®(guselkumab) injection ,for subcutaneous use
Initial U.S. Approval: 2017
Recent Major Changes
| Indications and Usage ( 1.2) | 07/2020 |
| Dosage and Administration ( 2.2) | 07/2020 |
| Warnings and Precautions, Hypersensitivity ( 5.1) | 06/2020 |
| Warnings and Precautions, Infections ( 5.2) | 07/2020 |
| Warnings and Precautions, Pre-treatment Evaluation for TB ( 5.3) | 07/2020 |
Indications and Usage for Tremfya
TREMFYA is an interleukin-23 blocker indicated for the treatment of adult patients with:
- moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy ( 1.1)
- active psoriatic arthritis. ( 1.2)
Tremfya Dosage and Administration
Plaque Psoriasis
100 mg administered by subcutaneous injection at Week 0, Week 4 and every 8 weeks thereafter. ( 2.1)
Psoriatic Arthritis
100 mg administered by subcutaneous injection at Week 0, Week 4 and every 8 weeks thereafter. TREMFYA can be used alone or in combination with a conventional DMARD (e.g. methotrexate). ( 2.2)
Dosage Forms and Strengths
Injection: 100 mg/mL in a single-dose prefilled syringe or single-dose One-Press patient-controlled injector. ( 3)
Contraindications
Serious hypersensitivity reactions to guselkumab or to any of the excipients. ( 4)
Warnings and Precautions
- Hypersensitivity Reactions: Serious hypersensitivity reactions, including anaphylaxis, may occur. ( 5.1)
- Infections: TREMFYA may increase the risk of infection. Instruct patients to seek medical advice if signs or symptoms of clinically important chronic or acute infection occur. If a serious infection develops, discontinue TREMFYA until the infection resolves. ( 5.2)
- Tuberculosis (TB): Evaluate for TB prior to initiating treatment with TREMFYA. ( 5.3)
Adverse Reactions/Side Effects
Most common (≥1%) adverse reactions associated with TREMFYA include upper respiratory infections, headache, injection site reactions, arthralgia, bronchitis, diarrhea, gastroenteritis, tinea infections, and herpes simplex infections. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Biotech, Inc. at 1-800-JANSSEN (1-800-526-7736) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 7/2020
Full Prescribing Information
1. Indications and Usage for Tremfya
2. Tremfya Dosage and Administration
2.1 Plaque Psoriasis
TREMFYA is administered by subcutaneous injection. The recommended dose is 100 mg at Week 0, Week 4, and every 8 weeks thereafter.
2.2 Psoriatic Arthritis
TREMFYA is administered by subcutaneous injection. The recommended dose is 100 mg at Week 0, Week 4, and every 8 weeks thereafter.
TREMFYA may be administered alone or in combination with a conventional disease-modifying antirheumatic drug (cDMARD) (e.g., methotrexate).
2.3 Important Administration Instructions
Administer TREMFYA subcutaneously. Each prefilled syringe or One-Press injector is for single-dose only. Instruct patients to inject the full amount (1 mL), which provides 100 mg of TREMFYA.
Do not inject TREMFYA into areas where the skin is tender, bruised, red, hard, thick, scaly, or affected by psoriasis [see Instructions for Use].
TREMFYA is intended for use under the guidance and supervision of a physician. TREMFYA may be administered by a health care professional, or a patient may self-inject after proper training in subcutaneous injection technique.
The TREMFYA Instructions for Usecontains more detailed patient instructions on the preparation and administration of TREMFYA [see Instructions for Use].
2.4 Preparation for Use of TREMFYA Prefilled Syringe or One-Press Injector
Before injection, remove TREMFYA prefilled syringe or One-Press injector from the refrigerator and allow TREMFYA to reach room temperature (30 minutes) without removing the needle cap.
Inspect TREMFYA visually for particulate matter and discoloration prior to administration. TREMFYA is a clear and colorless to light yellow solution that may contain small translucent particles. Do not use if the liquid contains large particles, is discolored or cloudy. TREMFYA does not contain preservatives; therefore, discard any unused product remaining in the prefilled syringe or One-Press injector.
3. Dosage Forms and Strengths
Injection: 100 mg/mL in a single-dose prefilled syringe or single-dose One-Press patient-controlled injector.
TREMFYA is a clear and colorless to light yellow solution that may contain small translucent particles.
4. Contraindications
TREMFYA is contraindicated in patients with a history of serious hypersensitivity reaction to guselkumab or to any of the excipients [see Warnings and Precautions (5.1)] .
5. Warnings and Precautions
5.1 Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis, have been reported with postmarket use of TREMFYA. Some cases required hospitalization. If a serious hypersensitivity reaction occurs, discontinue TREMFYA and initiate appropriate therapy.
5.2 Infections
TREMFYA may increase the risk of infection. In clinical trials in subjects with plaque psoriasis, infections occurred in 23% of subjects in the TREMFYA group versus 21% of subjects in the placebo group through 16 weeks of treatment. Upper respiratory tract infections, gastroenteritis, tinea infections, and herpes simplex infections occurred more frequently in the TREMFYA group than in the placebo group [see Adverse Reactions (6.1)] . The rate of serious infections for the TREMFYA group and the placebo group was ≤ 0.2%. A similar risk of infection was seen in placebo-controlled trials in subjects with psoriatic arthritis. Treatment with TREMFYA should not be initiated in patients with any clinically important active infection until the infection resolves or is adequately treated.
In patients with a chronic infection or a history of recurrent infection, consider the risks and benefits prior to prescribing TREMFYA. Instruct patients to seek medical help if signs or symptoms of clinically important chronic or acute infection occur. If a patient develops a clinically important or serious infection or is not responding to standard therapy, monitor the patient closely and discontinue TREMFYA until the infection resolves.
5.3 Pre-treatment Evaluation for Tuberculosis
Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with TREMFYA. Initiate treatment of latent TB prior to administering TREMFYA. In clinical trials, 105 subjects with plaque psoriasis and 71 subjects with psoriatic arthritis with latent TB who were concurrently treated with TREMFYA and appropriate TB prophylaxis did not develop active TB. Monitor patients for signs and symptoms of active TB during and after TREMFYA treatment. Consider anti-TB therapy prior to initiating TREMFYA in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Do not administer TREMFYA to patients with active TB infection.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of labeling:
- Infections [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Contraindications (4)and Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.2 Immunogenicity
As with all therapeutic proteins, there is the potential for immunogenicity with TREMFYA. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of incidence of antibodies to guselkumab across indications or with the incidences of antibodies to other products may be misleading.
6.3 Postmarketing Experience
The following adverse reactions have been reported during post-approval of TREMFYA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to TREMFYA exposure.
Immune system disorders:Hypersensitivity, including anaphylaxis [see Warnings and Precautions (5.1)]
Skin and subcutaneous tissue disorders:Rash [see Warnings and Precautions (5.1)]
7. Drug Interactions
7.1 CYP450 Substrates
The formation of CYP450 enzymes can be altered by increased levels of certain cytokines (e.g., IL-1, IL-6, IL-10, TNFα, interferon) during chronic inflammation.
Results from an exploratory drug-drug interaction study in subjects with moderate-to-severe plaque psoriasis suggested a low potential for clinically relevant drug interactions for drugs metabolized by CYP3A4, CYP2C9, CYP2C19 and CYP1A2 but the interaction potential cannot be ruled out for drugs metabolized by CYP2D6. However, the results were highly variable because of the limited number of subjects in the study.
Upon initiation of TREMFYA in patients who are receiving concomitant CYP450 substrates, particularly those with a narrow therapeutic index, consider monitoring for therapeutic effect or drug concentration and consider dosage adjustment as needed [see Clinical Pharmacology (12.3)] .
8. Use In Specific Populations
8.4 Pediatric Use
The safety and efficacy of TREMFYA in pediatric patients (less than 18 years of age) have not been established.
8.5 Geriatric Use
Of the 3406 subjects with plaque psoriasis or psoriatic arthritis exposed to TREMFYA, a total of 185 subjects were 65 years or older, and 13 subjects were 75 years or older. No overall differences in safety or effectiveness were observed between older and younger subjects who received TREMFYA. However, the number of subjects aged 65 years and older was not sufficient to determine whether they respond differently from younger subjects [see Clinical Pharmacology (12.3)] .
10. Overdosage
In the event of overdosage, monitor the patient for any signs or symptoms of adverse reactions and administer appropriate symptomatic treatment immediately.
11. Tremfya Description
Guselkumab, an interleukin-23 blocker, is a human immunoglobulin G1 lambda (IgG1λ) monoclonal antibody. Guselkumab is produced in a mammalian cell line using recombinant DNA technology.
TREMFYA (guselkumab) injection is a sterile, preservative free, clear, colorless to light yellow solution that may contain small translucent particles. TREMFYA is supplied as a single-dose solution in a 1 mL glass syringe with a 27G, half inch fixed needle assembled in a passive needle guard delivery system or One-Press patient-controlled injector for subcutaneous use.
Each TREMFYA 1 mL prefilled syringe or One-Press patient-controlled injector contains 100 mg guselkumab, L-histidine (0.6 mg), L-histidine monohydrochloride monohydrate (1.5 mg), polysorbate 80 (0.5 mg), sucrose (79 mg) and water for injection at pH 5.8.
12. Tremfya - Clinical Pharmacology
12.1 Mechanism of Action
Guselkumab is a human monoclonal IgG1λ antibody that selectively binds to the p19 subunit of interleukin 23 (IL-23) and inhibits its interaction with the IL-23 receptor. IL-23 is a naturally occurring cytokine that is involved in normal inflammatory and immune responses. Guselkumab inhibits the release of proinflammatory cytokines and chemokines.
12.2 Pharmacodynamics
In evaluated subjects with plaque psoriasis, guselkumab reduced serum levels of IL-17A, IL-17F and IL-22 relative to pre-treatment levels based on exploratory analyses of the pharmacodynamic markers.
In evaluated subjects with psoriatic arthritis, serum levels of acute phase proteins C-reactive protein, serum amyloid A and IL-6, and Th17 effector cytokines IL-17A, IL-17F and IL-22 were elevated at baseline. Serum levels of these proteins measured at Week 4 and Week 24 were decreased compared to baseline following guselkumab treatment at Week 0, Week 4 and every 8 weeks thereafter.
The relationship between these pharmacodynamic markers and the mechanism(s) by which guselkumab exerts its clinical effects is unknown.
12.3 Pharmacokinetics
Guselkumab exhibited linear pharmacokinetics in healthy subjects and subjects with plaque psoriasis following subcutaneous injections. In subjects with plaque psoriasis, following subcutaneous administration of 100 mg of TREMFYA at Weeks 0 and 4, and every 8 weeks thereafter, mean steady-state trough serum guselkumab concentration was approximately 1.2 mcg/mL.
The pharmacokinetics of guselkumab in subjects with psoriatic arthritis was similar to that in subjects with plaque psoriasis. Following subcutaneous administration of 100 mg of TREMFYA at Weeks 0, 4, and every 8 weeks thereafter, mean steady-state trough serum guselkumab concentration was approximately 1.2 mcg/mL.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies have not been conducted to evaluate the carcinogenic or mutagenic potential of TREMFYA.
No effects on fertility parameters were observed after male guinea pigs were subcutaneously administered guselkumab at a dose of 25 mg/kg twice weekly (15 times the MRHD based on a mg/kg comparison).
No effects on fertility parameters were observed after female guinea pigs were subcutaneously administered guselkumab at doses up to 100 mg/kg twice weekly (60 times the MRHD based on a mg/kg comparison).
14. Clinical Studies
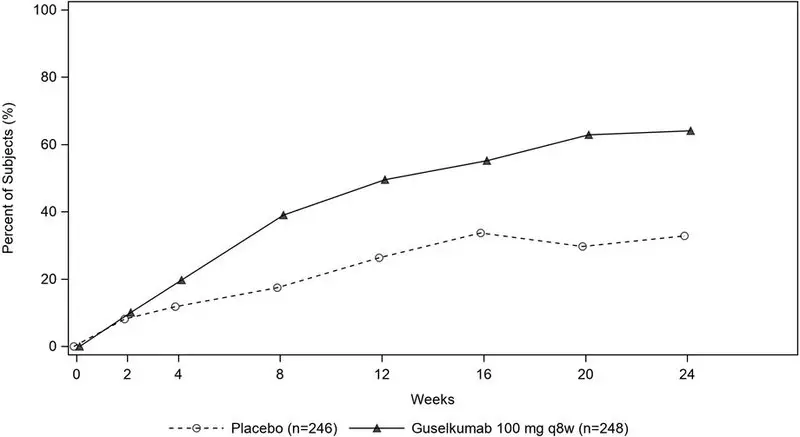
14.1 Plaque Psoriasis
Four multicenter, randomized, double-blind trials (PsO1 [NCT02207231], PsO2 [NCT02207244], PsO3 [NCT02203032], and PsO4 [NCT02905331]) enrolled subjects 18 years of age and older with moderate-to-severe plaque psoriasis who were eligible for systemic therapy or phototherapy. Subjects had an Investigator's Global Assessment (IGA) score of ≥3 ("moderate") on a 5-point scale of overall disease severity, a Psoriasis Area and Severity Index (PASI) score ≥12, and a minimum affected body surface area (BSA) of 10%. Subjects with guttate, erythrodermic, or pustular psoriasis were excluded.
14.2 Psoriatic Arthritis
The safety and efficacy of TREMFYA were assessed in 1120 subjects in 2 randomized, double-blind, placebo-controlled trials (PsA1 [NCT03162796] and PsA2 [NCT03158285]) in adult subjects with active psoriatic arthritis (PsA) (≥3 swollen joints, ≥3 tender joints, and a C-reactive protein (CRP) level of ≥0.3 mg/dL in PsA1 and ≥5 swollen joints, ≥5 tender joints, and a CRP level of ≥0.6 mg/dL in PsA2) who had inadequate response to standard therapies (e.g. conventional DMARDs [cDMARDs]), apremilast, or nonsteroidal anti-inflammatory drugs [NSAIDs]). Patients in these trials had a diagnosis of PsA for at least 6 months based on the Classification criteria for Psoriatic Arthritis (CASPAR) and a median duration of PsA of 4 years at baseline.
In PsA1 approximately 31% of subjects had been previously treated with up to 2 anti-tumor necrosis factor alpha (anti-TNFα) agents whereas in PsA2 all subjects were biologic naïve. Approximately 58% of subjects from both trials had concomitant methotrexate (MTX) use. Patients with different subtypes of PsA were enrolled in both trials, including polyarticular arthritis with the absence of rheumatoid nodules (40%), spondylitis with peripheral arthritis (30%), asymmetric peripheral arthritis (23%), distal interphalangeal involvement (7%) and arthritis mutilans (1%). At baseline, over 65% and 42% of the subjects had enthesitis and dactylitis, respectively and 79% had ≥3% body surface area (BSA) psoriasis skin involvement.
PsA1 evaluated 381 subjects who were treated with placebo SC, TREMFYA 100 mg SC at Weeks 0, 4 and every 8 weeks (q8w) thereafter, or TREMFYA 100 mg SC every 4 weeks (q4w). PsA2 evaluated 739 subjects who were treated with placebo SC, TREMFYA 100 mg SC at Weeks 0, 4 and q8w thereafter, or TREMFYA 100 mg SC q4w. The primary endpoint in both trials was the percentage of subjects achieving an ACR20 response at Week 24.
16. How is Tremfya supplied
16.1 How Supplied
TREMFYA (guselkumab) Injection is a clear and colorless to light yellow solution that may contain small translucent particles. TREMFYA is supplied as:
- Single-dose 100 mg/mL prefilled syringe (NDC: 57894-640-01)
- Single-dose 100 mg/mL One-Press patient-controlled injector (NDC: 57894-640-11)
16.2 Storage and Handling
TREMFYA is sterile and preservative-free. Discard any unused portion.
- Store in a refrigerator at 2ºC to 8ºC (36ºF to 46ºF).
- Store in original carton until time of use.
- Protect from light until use.
- Do not freeze.
- Do not shake.
- Not made with natural rubber latex.
Keep out of reach of children.
17. Patient Counseling Information
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use)before starting TREMFYA therapy, and each time the prescription is renewed, as there may be new information they need to know.
| Medication Guide
TREMFYA ®(trem fye' ah) (guselkumab) injection, for subcutaneous use |
|||||
|---|---|---|---|---|---|
| This Medication Guide had been approved by the U.S. Food and Drug Administration. | Revised: 07/2020 | ||||
| What is the most important information I should know about TREMFYA? | |||||
| TREMFYA may cause serious side effects, including: | |||||
|
|||||
|
|
||||
|
|||||
|
|
|
|||
| See " What are the possible side effects of TREMFYA?" for more information about side effects. | |||||
| What is TREMFYA? | |||||
| TREMFYA is a prescription medicine used to treat adults: | |||||
|
|||||
| It is not known if TREMFYA is safe and effective in children under 18 years of age. | |||||
| Do not use TREMFYAif you have had a serious allergic reaction to guselkumab or any of the other ingredients in TREMFYA. See the end of this Medication Guide for a complete list of ingredients in TREMFYA. | |||||
| Before using TREMFYA, tell your healthcare provider about all of your medical conditions, including if you: | |||||
|
|||||
| Tell your healthcare provider about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements. | |||||
| How should I use TREMFYA? | |||||
| See the detailed "Instructions for Use" that comes with TREMFYA for information on how to prepare and inject a dose of TREMFYA, and how to properly throw away (dispose of) used TREMFYA prefilled syringes or One-Press injectors. | |||||
|
|||||
| If you inject more TREMFYA than prescribed, call your healthcare provider right away. | |||||
| What are the possible side effects of TREMFYA? | |||||
| TREMFYA may cause serious side effects including: | |||||
|
|||||
| The most common side effects of TREMFYA include: | |||||
|
|
|
|||
| These are not all the possible side effects of TREMFYA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |||||
| General information about the safe and effective use of TREMFYA | |||||
| Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use TREMFYA for a condition for which it was not prescribed. Do not give TREMFYA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about TREMFYA that is written for health professionals. | |||||
| What are the ingredients in TREMFYA? | |||||
| Active ingredient:guselkumab | |||||
| Inactive ingredients:L-histidine, L-histidine monohydrochloride monohydrate, polysorbate 80, sucrose and water for injection | |||||
| Not made with natural rubber latex. | |||||
| Manufactured by: Janssen Biotech, Inc., Horsham, PA 19044, U.S. License Number 1864
For more information, call 1-800-526-7736 or go to www.tremfya.com. |
|||||
Instructions for Use
TREMFYA
®
(trem fye' ah)
(guselkumab)
Prefilled Syringe
SINGLE-DOSE
Important
TREMFYA comes as a single-dose prefilled syringe containing one 100 mg dose. Each TREMFYA prefilled syringe can only be used one time. Throw the used prefilled syringe away (See Step 3) after one dose, even if there is medicine left in it. Do not reuse your TREMFYA prefilled syringe.
If your healthcare provider decides that you or a caregiver may be able to give your injections of TREMFYA at home, you should receive training on the right way to prepare and inject TREMFYA using the prefilled syringe before attempting to inject. Do not try to inject yourself until you have been shown the right way to give the injections by your healthcare provider.
Read this Instructions for Use before using your TREMFYA prefilled syringe and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or your treatment.
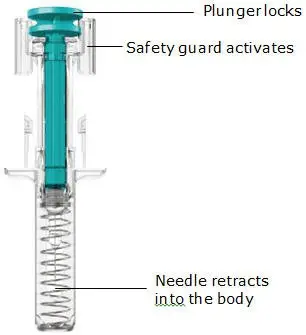
The TREMFYA prefilled syringe is intended for injection under the skin, not into the muscle or vein. After injection, the needle will retract into the body of the device and lock into place.
 Storage information
Storage information
Store in refrigerator at 36° to 46°F(2° to 8°C). Do notfreeze TREMFYA prefilled syringe.
Keep TREMFYA prefilled syringe and all medicines out of reach of children.
Do notshake your TREMFYA prefilled syringe.
Keep TREMFYA prefilled syringe in the original carton to protect from light and physical damage.
Prefilled syringe parts
Before use

After use
|
You will need these supplies:
Not provided in the TREMFYA prefilled syringe carton:
|
1. Prepare for your injection
Inspect carton
Remove your TREMFYA prefilled syringe carton from the refrigerator.
Keep the prefilled syringe in the carton and let it sit on a flat surface at room temperature for at least 30 minutesbefore use.
Do notwarm the prefilled syringe any other way.
Check the expiration date ('EXP')on the back panel of the carton.
Do notuse your prefilled syringe if the expiration date has passed.
Do notinject TREMFYA if the perforations on the carton are broken. Call your healthcare provider or pharmacist for a refill.
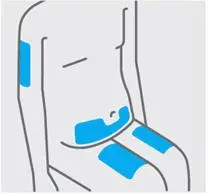
Choose injection site
Select from the following areas for your injection:
- Front of thighs(recommended)
- Lower stomach area (lower abdomen), except for a 2-inch area right around your navel (belly-button)
- Back of upper arms (only if someone else is giving you the injection)
Do notinject into skin that is tender, bruised, red, hard, thick, scaly or affected by psoriasis.
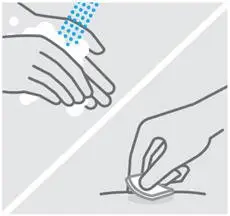
Clean injection site
Wash your hands well with soap and warm water.
Wipe your chosen injection site with an alcohol swab and allow it to dry.
Do nottouch, fan, or blow on the injection site after you have cleaned it.
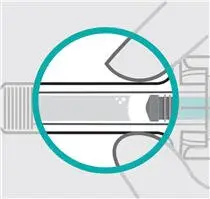
Inspect liquid
Take your TREMFYA prefilled syringe out of the carton.
Check the TREMFYA prefilled syringe liquid in the viewing window. It should be clear to slightly yellow and may contain tiny white or clear particles. You may also see one or more air bubbles. This is normal.
Do notinject if the liquid is cloudy or discolored, or has large particles. Call your healthcare provider or pharmacist for a refill.
2. Inject TREMFYA using prefilled syringe
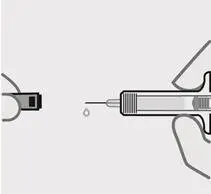
Remove needle cover
Hold your prefilled syringe by the body and pull needle cover straight off. It is normal to see a drop of liquid.
Inject TREMFYA within 5 minutes of removing the needle cover.
Do notput needle cover back on, as this may damage the needle or cause a needle stick injury.
Do nottouch needle or let it touch any surface.
Do notuse a TREMFYA prefilled syringe if it is dropped. Call your healthcare provider or pharmacist for a refill.
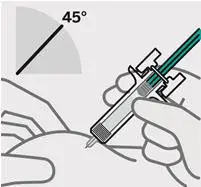
Position fingers and insert needle
Place your thumb, index and middle fingers directly under the finger flange, as shown.
Do nottouch plunger or area above finger flange as this may cause the needle safety device to activate.
Use your other hand to pinch skin at the injection site. Position syringe at about a 45 degree angle to the skin.
It is important to pinch enough skin to inject under the skinand not into the muscle.
Insert needle with a quick, dart-like motion.
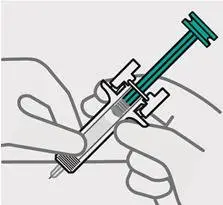
Release pinch and reposition hand
Use your free hand to grasp the body of the prefilled syringe.
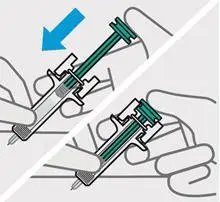
Press plunger
Place thumb from the opposite hand on the plunger and press the plunger all the way down until it stops.
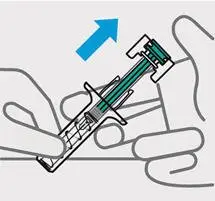
Release pressure from plunger
The safety guard will cover the needle and lock into place, removing the needle from your skin.
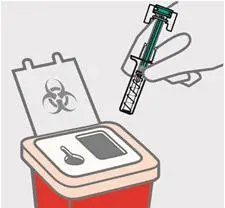
Dispose of your prefilled syringe
Put your used TREMFYA prefilled syringe in an FDA-cleared sharps disposal container right away after use.
Do notthrow away (dispose of) your TREMFYA prefilled syringe in your household trash.
Do notrecycle your used sharps disposal container.
For more information, see " How should I dispose of the used prefilled syringe?"
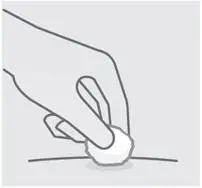
Check injection site
There may be a small amount of blood or liquid at the injection site. Hold pressure over your skin with a cotton ball or gauze pad until any bleeding stops.
Do notrub the injection site.
If needed, cover injection site with a bandage.
 Need help?
Need help?
Call your healthcare provider to talk about any questions you may have. For additional assistance or to share your feedback call 800-JANSSEN (800-526-7736).
How should I dispose of the used prefilled syringe?
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: www.fda.gov/safesharpsdisposal
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Janssen Biotech, Inc. Horsham, PA 19044
US License No. 1864
Approved: December 2017
INSTRUCTIONS FOR USE
TREMFYA
®
[trem fye´ ah]
(guselkumab)
injection, for subcutaneous use
One-Press Patient-Controlled Injector
This "Instructions for Use" contains information on how to inject TREMFYA.
Important Information You Need to Know Before Injecting TREMFYA
TREMFYA comes in a single-dose One-Press injector containing one 100 mg dose.
| During injection, push handle all the way down until teal body is not visible to inject the full dose.
DO NOT LIFT ONE-PRESS during injection. If you do, the One-Press will lock and you will not get the full dose. |
Each One-Press injector can only be used one time. Throw away (See Step 3) after one dose, even if there is medicine left in it. Do not reuse your One-Press injector.
If your healthcare provider decides that you or a caregiver may be able to give your injections of TREMFYA at home, you should receive training on the right way to prepare and inject TREMFYA using the One-Press injector. Do not try to inject yourself until you have been trained by your healthcare provider.
Please read this Instructions for Use before using your One-Press injector and each time you get a new One-Press injector. There may be new information.
This leaflet does not take the place of talking with your healthcare provider about your medical condition or your treatment.
 Storage information
Storage information
Store in refrigerator at 36° to 46°F(2° to 8°C).
Do notfreeze your One-Press injector.
Do notshake your One-Press injector.
Keep your One-Press injector and all medicines out of reach of children.
Keep your One-Press injector in the original carton to protect from light and physical damage.
 Need help?
Need help?
Call your healthcare provider to talk about any questions you may have. For additional assistance or to share your feedback call 800-JANSSEN (800-526-7736).
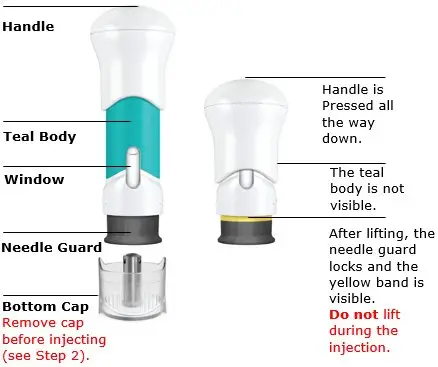
One-Press injector parts
Before use After use
You will need:
|
1. Preparing to Inject TREMFYA
Inspect carton and allow TREMFYA to come to room temperature
Remove your One-Press injector carton from the refrigerator.
Keep your One-Press injector in the carton and let it sit on a flat surface at room temperature for at least 30 minutesbefore use.
Do notwarm your One-Press injector any other way.
Check the expiration date ('EXP') on the carton
Do notuse your One-Press injector if the expiration date has passed.
Do notinject TREMFYA if the seal on the carton is broken. Call your healthcare provider or pharmacist for a new One-Press injector.
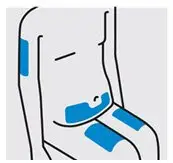
Choose injection site
Select from the following areas for your injection:
- Front of thighs(recommended)
- Lower stomach area (lower abdomen), except for a 2-inch area right around your navel (belly-button)
- Back of upper arms (only if someone else is giving you the injection)
Do notinject into skin that is tender, bruised, red, hard, thick, scaly, or affected by psoriasis.
Wash hands
Wash your hands well with soap and warm water.
Clean injection site
Wipe your chosen injection site with an alcohol swab and allow it to dry.
Do nottouch, fan, or blow on the injection site after you have cleaned it.
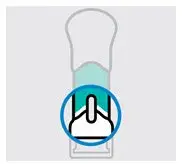
Inspect liquid in window
Take your One-Press injector out of the carton.
Check the liquid in the window. It should be clear to slightly yellow and may contain tiny white or clear particles. You may also see one or more air bubbles. This is normal.
Do notinject if the liquid is:
- cloudy,
- discolored, or
- has large particles.
Call your healthcare provider or pharmacist for a new One-Press injector.
2. Injecting TREMFYA
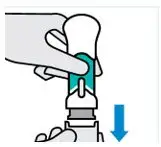
Pull off bottom cap
Keep hands away from the needle guard after the cap is removed. It is normal to see a few drops of liquid.
Inject TREMFYA within 5 minutes of removing the cap.
Do notput the cap back on. This could damage the needle.
Do notuse a One-Press injector if it is dropped after removing the cap. Call your healthcare provider or pharmacist for a new One-Press injector.
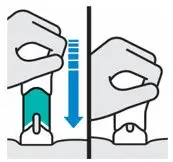
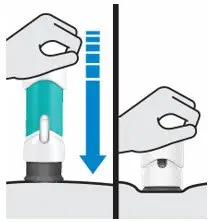
Place straight on skin.
Push handle all the way down until teal body is not visible
| DO NOT LIFT ONE-PRESS during injection!
If you do, the needle guard will lock, showing a yellow band, and you will not get the full dose. |
You may hear a click when the injection begins. Keep pushing.
If you feel resistance, keep pushing. This is normal.
The medication injects as you push. Do this at a speed that is comfortable for you.
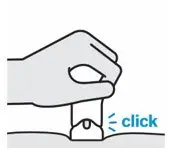
Confirm your injection is complete
Your injection is complete when:
- The teal body is not visible.
- You cannot press the handle down anymore.
- You may hear a click.
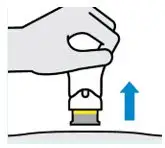
Lift straight up
The yellow band indicates that the needle guard is locked.
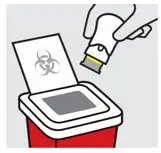
Dispose of your One-Press injector
Put your used One-Press injector in a sharps disposal container right away after use.
Do notthrow away (dispose of) your One-Press injector in your household trash.
Do notrecycle your used sharps disposal container.
For more information, see " Disposing of TREMFYA One-Press injector".
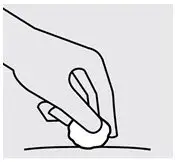
Check injection site
There may be a small amount of blood or liquid at the injection site. Hold pressure over your skin with a cotton ball or gauze pad until any bleeding stops.
Do notrub the injection site.
If needed, cover injection site with a bandage.
Disposing of TREMFYA One-Press injector
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: www.fda.gov/safesharpsdisposal. You may also consult your pharmacist.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Janssen Biotech, Inc. Horsham, PA 19044
US License No. 1864
Revised: June 2023
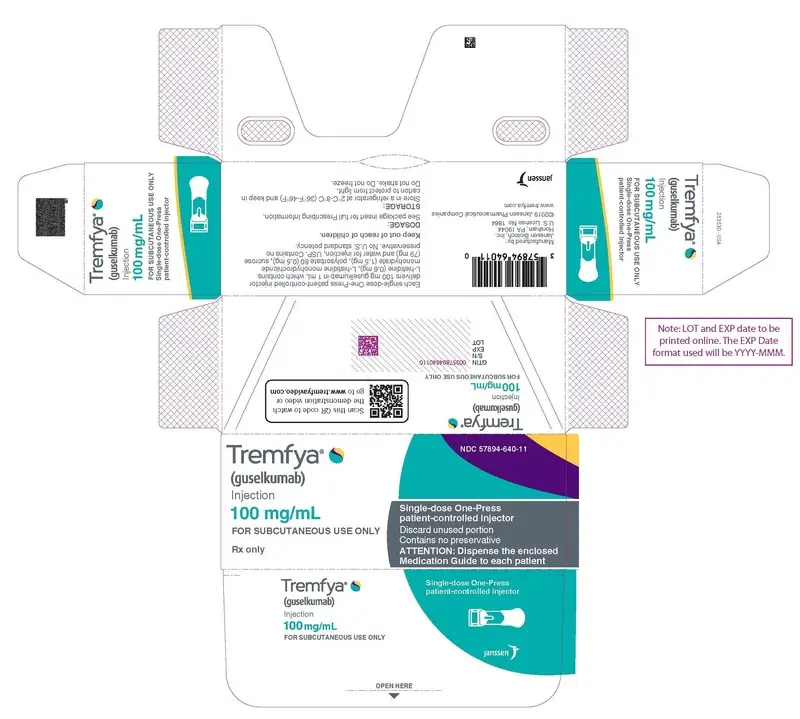
PRINCIPAL DISPLAY PANEL - 100 mg/mL Syringe Carton
Tremfya
®
(guselkumab)
Injection
100 mg/mL
FOR SUBCUTANEOUS USE ONLY
Rx only
NDC 57894-640-11
Single-dose One-Press
patient-controlled injector
Discard unused portion
Contains no preservative
ATTENTION: Dispense the enclosed
Medication Guide to each patient
| TREMFYA
guselkumab injection |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Janssen Biotech, Inc. (099091753) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Biotech, Inc. | 038978363 | api manufacture(57894-640) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AndersonBrecon Inc. | 053217022 | pack(57894-640) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Biologics B.V. | 409612918 | analysis(57894-640) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cilag AG | 483237103 | manufacture(57894-640) , analysis(57894-640) , pack(57894-640) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Biogen MA Inc. | 841087823 | api manufacture(57894-640) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PPD Development Ireland Ltd. | 985036175 | analysis(57894-640) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Sciences Ireland UC | 986030167 | api manufacture(57894-640) , analysis(57894-640) | |