Drug Detail:Tukysa (Tucatinib [ tu-kat-e-nib ])
Drug Class: HER2 inhibitors
Highlights of Prescribing Information
TUKYSA® (tucatinib) tablets, for oral use
Initial U.S. Approval: 2020
Recent Major Changes
| Indications and Usage (1.2) | 1/2023 |
| Patient Selection (2.1) | 1/2023 |
| Warnings and Precautions (5.1), (5.2) | 1/2023 |
Indications and Usage for Tukysa
TUKYSA is a kinase inhibitor indicated:
- in combination with trastuzumab and capecitabine for treatment of adult patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who have received one or more prior anti-HER2-based regimens in the metastatic setting. (1.1)
- in combination with trastuzumab for the treatment of adult patients with RAS wild-type HER2-positive unresectable or metastatic colorectal cancer that has progressed following treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy. (1.2)
This indication is approved under accelerated approval based on tumor response rate and durability of response [see Clinical Studies (14.2)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Tukysa Dosage and Administration
- In patients with unresectable or metastatic colorectal cancer, confirm the presence of HER2 protein overexpression and RAS wild-type in tumor specimens prior to the initiation of TUKYSA (2.1)
- Recommended dosage: 300 mg taken orally twice daily with or without food. (2.1)
- For patients with severe hepatic impairment, the recommended dosage is 200 mg orally twice daily. (2.3, 8.7)
Dosage Forms and Strengths
Tablets: 50 mg and 150 mg. (3)
Contraindications
None. (4)
Warnings and Precautions
-
Diarrhea: Severe diarrhea, including dehydration, acute kidney injury, and death, has been reported. Administer antidiarrheal treatment as clinically indicated. Interrupt dose, then dose reduce, or permanently discontinue TUKYSA based on severity. (2.2, 5.1)
- Hepatotoxicity: Severe hepatotoxicity has been reported on TUKYSA. Monitor ALT, AST and bilirubin prior to starting TUKYSA, every 3 weeks during treatment and as clinically indicated. Interrupt dose, then dose reduce, or permanently discontinue TUKYSA based on severity. (2.2, 5.2)
-
Embryo-Fetal Toxicity: TUKYSA can cause fetal harm. Advise patients of potential risk to a fetus and to use effective contraception. (5.3, 8.1, 8.3)
Also, refer to the Full Prescribing Information of trastuzumab and capecitabine for pregnancy and contraception information.
Adverse Reactions/Side Effects
- The most common adverse reactions (≥20%) with TUKYSA in combination with trastuzumab and capecitabine in patients with metastatic breast cancer are diarrhea, palmar-plantar erythrodysesthesia, nausea, hepatotoxicity, vomiting, stomatitis, decreased appetite, anemia, and rash. (6.1)
- The most common adverse reactions (≥20%) with TUKYSA in combination with trastuzumab in patients with unresectable or metastatic colorectal cancer are diarrhea, fatigue, rash, nausea, abdominal pain, infusion related reactions, and pyrexia.
To report SUSPECTED ADVERSE REACTIONS, contact Seagen at 1-855-4SEAGEN or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Strong CYP3A Inducers or Moderate CYP2C8 Inducers: Avoid concomitant use. (7.1)
- Strong CYP2C8 Inhibitors: Avoid concomitant use; reduce TUKYSA dose if concomitant use cannot be avoided. (2.4, 7.1)
- CYP3A Substrates: Avoid concomitant use with CYP3A substrates, where minimal concentration changes may lead to serious or life-threatening toxicities. (7.2)
-
P-gp Substrates: Consider reducing the dose of P-gp substrates, where minimal concentration changes may lead to serious or life-threatening toxicities. (7.2)
Use In Specific Populations
Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2023
Related/similar drugs
Keytruda, Trodelvy, Arimidex, Avastin, capecitabine, fluorouracil, FemaraFull Prescribing Information
1. Indications and Usage for Tukysa
1.1 Metastatic Breast Cancer
TUKYSA is indicated in combination with trastuzumab and capecitabine for treatment of adult patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who have received one or more prior anti-HER2-based regimens in the metastatic setting.
1.2 Unresectable or Metastatic Colorectal Cancer
TUKYSA is indicated in combination with trastuzumab for the treatment of adult patients with RAS wild-type, HER2-positive unresectable or metastatic colorectal cancer that has progressed following treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy.
This indication is approved under accelerated approval based on tumor response rate and durability of response [see Clinical Studies (14.2)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
2. Tukysa Dosage and Administration
2.1 Patient Selection
Select patients for treatment of unresectable or metastatic colorectal cancer with TUKYSA based on the presence of:
- HER2 overexpression or gene amplification [see Clinical Studies (14.2)]. FDA-approved tests for the detection of HER2 overexpression and gene amplification in patients with unresectable or metastatic colorectal cancer are not currently available, and
- RAS wild-type [see Clinical Studies (14.2)]. Information on FDA-approved tests for the detection of RAS mutations in patients with unresectable or metastatic colorectal cancer is available at http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dosage
Metastatic Breast Cancer
The recommended dosage of TUKYSA is 300 mg taken orally twice daily in combination with trastuzumab and capecitabine until disease progression or unacceptable toxicity [see Clinical Studies (14.1)].
Unresectable or Metastatic Colorectal Cancer
The recommended dosage of TUKYSA is 300 mg taken orally twice daily in combination with trastuzumab until disease progression or unacceptable toxicity [see Clinical Studies (14.2)].
Advise patients to swallow TUKYSA tablets whole and not to chew, crush, or split prior to swallowing. Advise patients not to ingest tablet if it is broken, cracked, or not otherwise intact.
Advise patients to take TUKYSA approximately 12 hours apart and at the same time each day with or without a meal.
If the patient vomits or misses a dose of TUKYSA, instruct the patient to take the next dose at its usual scheduled time.
When given in combination with TUKYSA, the recommended dosage of capecitabine is 1000 mg/m2 orally twice daily taken within 30 minutes after a meal. TUKYSA and capecitabine can be taken at the same time. Refer to the Full Prescribing Information for trastuzumab and capecitabine for additional information.
2.3 Dosage Modifications for Adverse Reactions
The recommended TUKYSA dose reductions and dosage modifications for adverse reactions are provided in Tables 1 and 2. Refer to the Full Prescribing Information for trastuzumab and capecitabine for information about dosage modifications for these drugs.
| Dose Reduction | Recommended TUKYSA Dosage |
| First | 250 mg orally twice daily |
| Second | 200 mg orally twice daily |
| Third | 150 mg orally twice daily |
Permanently discontinue TUKYSA in patients unable to tolerate 150 mg orally twice daily.
| 1. Grades based on National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.03 2. Abbreviations: ULN = upper limit of normal; ALT = alanine aminotransferase; AST = aspartate aminotransferase |
||
| Adverse Reaction1 | Severity | TUKYSA Dosage Modification |
| Diarrhea [see Warnings and Precautions (5.1)] | Grade 3 without anti-diarrheal treatment | Initiate or intensify appropriate medical therapy. Hold TUKYSA until recovery to ≤ Grade 1, then resume TUKYSA at the same dose level. |
| Grade 3 with anti-diarrheal treatment | Initiate or intensify appropriate medical therapy. Hold TUKYSA until recovery to ≤ Grade 1, then resume TUKYSA at the next lower dose level. | |
| Grade 4 | Permanently discontinue TUKYSA. | |
| Hepatotoxicity2 [see Warnings and Precautions (5.2)] | Grade 2 bilirubin (>1.5 to 3 × ULN) | Hold TUKYSA until recovery to ≤ Grade 1, then resume TUKYSA at the same dose level. |
| Grade 3 ALT or AST (> 5 to 20 × ULN) OR Grade 3 bilirubin (> 3 to 10 × ULN) | Hold TUKYSA until recovery to ≤ Grade 1, then resume TUKYSA at the next lower dose level. | |
| Grade 4 ALT or AST (> 20 × ULN) OR Grade 4 bilirubin (> 10 × ULN) | Permanently discontinue TUKYSA. | |
| ALT or AST > 3 × ULN AND Bilirubin > 2 × ULN | Permanently discontinue TUKYSA. | |
| Other adverse reactions [see Adverse Reactions (6.1)] | Grade 3 | Hold TUKYSA until recovery to ≤ Grade 1, then resume TUKYSA at the next lower dose level. |
| Grade 4 | Permanently discontinue TUKYSA. | |
2.4 Dosage Modifications for Severe Hepatic Impairment
For patients with severe hepatic impairment (Child-Pugh C), reduce the recommended dosage to 200 mg orally twice daily [see Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
2.5 Dosage Modifications for Concomitant Use with Strong CYP2C8 Inhibitors
Avoid concomitant use of strong CYP2C8 inhibitors with TUKYSA. If concomitant use with a strong CYP2C8 inhibitor cannot be avoided, reduce the recommended dosage to 100 mg orally twice daily. After discontinuation of the strong CYP2C8 inhibitor for 3 elimination half-lives, resume the TUKYSA dose that was taken prior to initiating the inhibitor [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
Tablets:
- 50 mg: round, yellow, film-coated, debossed with “TUC” on one side and “50” on the other side.
- 150 mg: oval-shaped, yellow, film-coated, debossed with “TUC” on one side and “150” on the other side.
5. Warnings and Precautions
5.1 Diarrhea
TUKYSA can cause severe diarrhea including dehydration, hypotension, acute kidney injury, and death [see Adverse Reactions (6.1)].
If diarrhea occurs, administer antidiarrheal treatment as clinically indicated. Perform diagnostic tests as clinically indicated to exclude other causes of diarrhea. Based on the severity of the diarrhea, interrupt dose, then dose reduce or permanently discontinue TUKYSA [see Dosage and Administration (2.2)].
TUKYSA with trastuzumab and capecitabine
In HER2CLIMB, 81% of patients who received TUKYSA experienced diarrhea, including 0.5% with Grade 4 diarrhea and 12% with Grade 3 diarrhea. Both patients who developed Grade 4 diarrhea subsequently died, with diarrhea as a contributor to death. The median time to onset of the first episode of diarrhea was 12 days and the median time to resolution was 8 days. Diarrhea led to dose reductions of TUKYSA in 6% of patients and discontinuation of TUKYSA in 1% of patients. Prophylactic use of antidiarrheal treatment was not required on HER2CLIMB.
TUKYSA with trastuzumab
In MOUNTAINEER, diarrhea occurred in 64% of patients, including Grade 3 (3.5%), Grade 2 (10%), and Grade 1 (50%).
5.2 Hepatotoxicity
TUKYSA can cause severe hepatotoxicity [see Adverse Reactions (6.1)].
Monitor ALT, AST, and bilirubin prior to starting TUKYSA, every 3 weeks during treatment, and as clinically indicated. Based on the severity of hepatotoxicity, interrupt dose, then dose reduce or permanently discontinue TUKYSA [see Dosage and Administration (2.2)].
TUKYSA with trastuzumab and capecitabine
In HER2CLIMB, 8% of patients who received TUKYSA had an ALT increase > 5 × ULN, 6% had an AST increase > 5 × ULN, and 1.5% had a bilirubin increase > 3 × ULN (Grade ≥3). Hepatotoxicity led to dose reduction of TUKYSA in 8% of patients and discontinuation of TUKYSA in 1.5% of patients.
TUKYSA with trastuzumab
In MOUNTAINEER, 6% of patients had a bilirubin increase > 3 × ULN (Grade ≥3), 6% had an AST increase > 5 × ULN, and 4.7% had an ALT increase > 5 × ULN. Hepatotoxicity led to dose reduction of TUKYSA in 3.5% of patients and discontinuation of TUKYSA in 2.3% of patients.
5.3 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, TUKYSA can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of tucatinib to pregnant rats and rabbits during organogenesis caused embryo-fetal mortality, reduced fetal weight and fetal abnormalities at maternal exposures ≥ 1.3 times the human exposure (AUC) at the recommended dose.
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with TUKYSA and for 1 week after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with TUKYSA and for 1 week after the last dose [see Use in Specific Populations (8.1, 8.3)].
TUKYSA is used in combination with trastuzumab and capecitabine. Refer to the Full Prescribing Information of trastuzumab and capecitabine for pregnancy and contraception information.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Diarrhea [see Warnings and Precautions (5.1)]
- Hepatotoxicity [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
HER2-Positive Metastatic Breast Cancer
The safety of TUKYSA in combination with trastuzumab and capecitabine was evaluated in HER2CLIMB [see Clinical Studies (14)]. Patients received either TUKYSA 300 mg twice daily plus trastuzumab or a non-US approved trastuzumab product, and capecitabine (n=404) or placebo plus trastuzumab or a non-US approved trastuzumab product and capecitabine (n=197). The median duration of treatment was 5.8 months (range: 3 days, 2.9 years) for the TUKYSA arm.
Serious adverse reactions occurred in 26% of patients who received TUKYSA. Serious adverse reactions in ≥ 2% of patients who received TUKYSA were diarrhea (4%), vomiting (2.5%), nausea (2%), abdominal pain (2%), and seizure (2%). Fatal adverse reactions occurred in 2% of patients who received TUKYSA including sudden death, sepsis, dehydration, and cardiogenic shock.
Adverse reactions leading to treatment discontinuation occurred in 6% of patients who received TUKYSA. Adverse reactions leading to treatment discontinuation of TUKYSA in ≥1% of patients were hepatotoxicity (1.5%) and diarrhea (1%).
Adverse reactions leading to dose reduction occurred in 21% of patients who received TUKYSA. Adverse reactions leading to dose reduction of TUKYSA in ≥2% of patients were hepatotoxicity (8%) and diarrhea (6%).
The most common adverse reactions in patients who received TUKYSA (≥20%) were diarrhea, palmar-plantar erythrodysesthesia, nausea, hepatotoxicity, vomiting, stomatitis, decreased appetite, anemia, and rash.
Table 3 summarizes the adverse reactions in HER2CLIMB.
|
||||||
| Adverse Reaction | TUKYSA + Trastuzumab + Capecitabine N = 404 | Placebo + Trastuzumab + Capecitabine N = 197 |
||||
| All Grades% | Grade 3% | Grade 4% | All Grades% | Grade 3% | Grade 4% | |
| Gastrointestinal disorders | ||||||
| Diarrhea | 81 | 12 | 0.5 | 53 | 9 | 0 |
| Nausea | 58 | 3.7 | 0 | 44 | 3 | 0 |
| Vomiting | 36 | 3 | 0 | 25 | 3.6 | 0 |
| Stomatitis1 | 32 | 2.5 | 0 | 21 | 0.5 | 0 |
| Skin and subcutaneous tissue disorders | ||||||
| Palmar-plantar erythrodysesthesia syndrome | 63 | 13 | 0 | 53 | 9 | 0 |
| Rash2 | 20 | 0.7 | 0 | 15 | 0.5 | 0 |
| Hepatobiliary disorders | ||||||
| Hepatotoxicity3 | 42 | 9 | 0.2 | 24 | 3.6 | 0 |
| Metabolism and nutrition disorders | ||||||
| Decreased appetite | 25 | 0.5 | 0 | 20 | 0 | 0 |
| Blood and lymphatic system disorders | ||||||
| Anemia4 | 21 | 3.7 | 0 | 13 | 2.5 | 0 |
| Musculoskeletal and connective tissue disorders | ||||||
| Arthralgia | 15 | 0.5 | 0 | 4.6 | 0.5 | 0 |
| Investigations | ||||||
| Creatinine increased5 | 14 | 0 | 0 | 1.5 | 0 | 0 |
| Weight decreased | 13 | 1 | 0 | 6 | 0.5 | 0 |
| Nervous System Disorders | ||||||
| Peripheral neuropathy6 | 13 | 0.5 | 0 | 7 | 1 | 0 |
| Respiratory, thoracic and mediastinal disorders | ||||||
| Epistaxis | 12 | 0 | 0 | 5 | 0 | 0 |
|
||||
| Laboratory Abnormality | TUKYSA + Trastuzumab + Capecitabine1 | Placebo + Trastuzumab + Capecitabine1 |
||
| All Grades % | Grades ≥3 % | All Grades % | Grades ≥ 3% |
|
| Hematology | ||||
| Decreased hemoglobin | 59 | 3.3 | 51 | 1.5 |
| Chemistry | ||||
| Decreased phosphate | 57 | 8 | 45 | 7 |
| Increased bilirubin | 47 | 1.5 | 30 | 3.1 |
| Increased ALT | 46 | 8 | 27 | 0.5 |
| Increased AST | 43 | 6 | 25 | 1 |
| Decreased magnesium | 40 | 0.8 | 25 | 0.5 |
| Decreased potassium 2 | 36 | 6 | 31 | 5 |
| Increased creatinine 3 | 33 | 0 | 6 | 0 |
| Decreased sodium 4 | 28 | 2.5 | 23 | 2 |
| Increased alkaline phosphatase | 26 | 0.5 | 17 | 0 |
Increased Creatinine
The mean increase in serum creatinine was 32% within the first 21 days of treatment with TUKYSA. The serum creatinine increases persisted throughout treatment and were reversible upon treatment completion. Consider alternative markers of renal function if persistent elevations in serum creatinine are observed [see Clinical Pharmacology (12.3)].
RAS Wild-Type, HER2-Positive Unresectable or Metastatic Colorectal Cancer
The safety of TUKYSA in combination with trastuzumab or a non-US approved trastuzumab product was evaluated in 86 patients enrolled in MOUNTAINEER with unresectable or metastatic colorectal cancer [see Clinical Studies (14.2)]. The median duration of exposure to TUKYSA was 6.9 months (range 0.7, 49.3).
Serious adverse reactions occurred in 22% of patients. Serious adverse reactions that occurred in ≥ 2% of patients were intestinal obstruction (7%), urinary tract infection (3.5%), pneumonia (2.3%), abdominal pain (2.3%) and rectal perforation (2.3%).
Permanent discontinuation of TUKYSA due to an adverse reaction occurred in 6% of patients. The adverse reaction which resulted in permanent discontinuation of TUKYSA in ≥2% of patients was increased ALT (2.3%). Dosage interruptions of TUKYSA due to an adverse reaction occurred in 23% of patients. Adverse reactions which required dosage interruption in ≥3% of patients were increased ALT (3.5%) and diarrhea (3.5%). Dose reductions of TUKYSA due to an adverse reaction occurred in 9% of patients. Adverse reactions which required dose reductions in ≥2% of patients were increased ALT (2.3%) and diarrhea (2.3%).
The most common adverse reactions reported in ≥ 20% of patients treated with TUKYSA and trastuzumab were diarrhea, fatigue, rash, nausea, abdominal pain, infusion related reactions, and pyrexia. The most common laboratory abnormalities reported in ≥ 20% of patients were increased creatinine, increased glucose, increased ALT, decreased hemoglobin, increased AST, increased bilirubin, increased alkaline phosphatase, decreased lymphocytes, decreased albumin, decreased leukocytes, and decreased sodium.
Table 5 summarizes the adverse reactions in MOUNTAINEER.
| *Includes 1 patient who only received trastuzumab. 1. Abdominal pain includes abdominal discomfort, abdominal pain, and abdominal pain upper. 2. Rash includes acne, dermatitis acneiform, dermatitis contact, erythema, erythema multiforme, rash, rash macular, rash maculo-papular, rash papular, rash pustular, skin exfoliation, and urticaria, |
||
|
Adverse Reaction | TUKYSA + Trastuzumab N= 86* |
|
| All Grades% | Grade 3% | |
| Gastrointestinal disorders | ||
| Diarrhea | 64 | 3.5 |
| Nausea | 35 | 0 |
| Vomiting | 16 | 0 |
| Abdominal pain1 | 21 | 2.3 |
| Constipation | 14 | 1.2 |
| General disorders | ||
| Fatigue | 44 | 2.3 |
| Pyrexia | 20 | 0 |
| Chills | 19 | 1.2 |
| Skin and subcutaneous disorders | ||
| Rash2 | 37 | 0 |
| Injury, poisoning, and procedural complications | ||
| Infusion related reaction | 21 | 0 |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 19 | 0 |
| Blood and lymphatic system disorders | ||
| Anemia | 10 | 0 |
| Vascular disorders |
|
|
| Hypertension | 17 | 7 |
| Musculoskeletal and connective tissue disorders | ||
| Back pain | 17 | 2.3 |
| Arthralgia | 16 | 1.2 |
| Myalgia | 13 | 0 |
| Respiratory, thoracic and mediastinal disorders | ||
| Cough | 16 | 0 |
| Dyspnea | 14 | 0 |
| Psychiatric disorders | ||
| Anxiety | 10 | 0 |
Other adverse reactions (<10%) include epistaxis (7%), weight decreased (7%), oropharyngeal pain (5%), oral dysesthesia (1%), and stomatitis (1%).
| 1. Number of patients with both baseline and post-baseline results for each test 2. NCI CTCAE v5.0 was used for creatinine increased. NCI CTCAE v4.03 was used for all other laboratory parameters. 3. Due to inhibition of renal tubular transport of creatinine without affecting glomerular function |
|||
| Laboratory Abnormality | TUKYSA + Trastuzumab N=851 |
||
| All Grades% | Grade 3% | Grade 4% | |
| Hematology | |||
| Decreased hemoglobin | 46 | 3.5 | 0 |
| Decreased lymphocytes | 39 | 12 | 0 |
| Decreased leukocytes | 22 | 0 | 0 |
| Decreased platelets | 15 | 0 | 0 |
| Chemistry | |||
| Increased creatinine2,3 | 58 | 0 | 0 |
| Increased glucose | 56 | 2.4 | 0 |
| Increased ALT | 46 | 2.4 | 2.4 |
| Increased AST | 33 | 2.4 | 3.5 |
| Increased bilirubin | 28 | 3.5 | 2.4 |
| Increased alkaline phosphatase | 25 | 1.2 | 0 |
| Decreased albumin | 24 | 1.2 | 0 |
| Decreased sodium | 20 | 6 | 0 |
| Decreased potassium | 16 | 1.2 | 0 |
Increased Creatinine
The mean increase in serum creatinine was 32% within the first 21 days of treatment with TUKYSA. The serum creatinine increases persisted throughout treatment and were reversible in 87% of patients with values outside normal lab limits upon treatment completion. Consider alternative markers of renal function if persistent elevations in serum creatinine are observed [see Clinical Pharmacology (12.3)].
7. Drug Interactions
7.1 Effects of Other Drugs on TUKYSA
Table 7 summarizes the effect of other drugs on TUKYSA.
| Strong CYP3A Inducers or Moderate CYP2C8 Inducers | |
| Clinical Impact | Concomitant use of TUKYSA with a strong CYP3A or moderate CYP2C8 inducer decreased tucatinib plasma concentrations [see Clinical Pharmacology (12.3)] which may reduce TUKYSA activity. |
| Management | Avoid concomitant use of TUKYSA with a strong CYP3A inducer or a moderate CYP2C8 inducer. |
| Strong or Moderate CYP2C8 Inhibitors | |
| Clinical Impact | Concomitant use of TUKYSA with a strong CYP2C8 inhibitor increased tucatinib plasma concentrations [see Clinical Pharmacology (12.3)], which may increase the risk of TUKYSA toxicity. |
| Management | Avoid concomitant use of TUKYSA with a strong CYP2C8 inhibitor. Increase monitoring for TUKYSA toxicity with moderate CYP2C8 inhibitors. |
7.2 Effects of TUKYSA on Other Drugs
Table 8 summarizes the effect of TUKYSA on other drugs.
| CYP3A Substrates | |
| Clinical Impact | Concomitant use of TUKYSA with a CYP3A substrate increased the plasma concentrations of CYP3A substrate [see Clinical Pharmacology (12.3)], which may increase the toxicity associated with a CYP3A substrate. |
| Management | Avoid concomitant use of TUKYSA with CYP3A substrates, where minimal concentration changes may lead to serious or life-threatening toxicities. If concomitant use is unavoidable, decrease the CYP3A substrate dosage in accordance with approved product labeling. |
| P-glycoprotein (P-gp) Substrates | |
| Clinical Impact | Concomitant use of TUKYSA with a P-gp substrate increased the plasma concentrations of P-gp substrate [see Clinical Pharmacology (12.3)], which may increase the toxicity associated with a P-gp substrate. |
| Management | Consider reducing the dosage of P-gp substrates, where minimal concentration changes may lead to serious or life-threatening toxicities. |
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
TUKYSA is used in combination with trastuzumab and capecitabine. Refer to the Full Prescribing Information of trastuzumab and capecitabine for pregnancy information.
Based on findings in animals and its mechanism of action, TUKYSA can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available human data on TUKYSA use in pregnant women to inform a drug-associated risk. In animal reproduction studies, administration of tucatinib to pregnant rats and rabbits during organogenesis resulted in embryo-fetal mortality, reduced fetal weight and fetal abnormalities at maternal exposures ≥ 1.3 times the human exposure (AUC) at the recommended dose (see Data). Advise pregnant women and females of reproductive potential of the potential risk to the fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Animal Data
In pilot embryo-fetal development studies, pregnant rats and rabbits received oral doses of tucatinib up to 150 mg/kg/day during the period of organogenesis.
In rats, oral administration of tucatinib resulted in maternal toxicity (body weight loss, reduced body weight gain, low food consumption) at doses ≥ 90 mg/kg/day. Fetal effects included reduced number of live fetuses, decreased fetal weight, and fetal abnormalities (increase in skeletal variations, incomplete ossification) at ≥ 90 mg/kg/day (approximately 3.5 times the human exposure at the recommended dose based on AUC).
In rabbits, oral administration of tucatinib resulted in increased resorptions, decreased percentages of live fetuses, and skeletal, visceral, and external malformations in fetuses at doses ≥ 90 mg/kg/day (1.3 times the human exposure at the recommended dose based on AUC). Fetal abnormalities included domed head, brain dilation, incomplete ossification of frontal and parietal bones, and a hole in the parietal bone.
8.2 Lactation
Risk Summary
TUKYSA is used in combination with trastuzumab and capecitabine. Refer to the Full Prescribing Information of trastuzumab and capecitabine for lactation information.
There are no data on the presence of tucatinib or its metabolites in human or animal milk or its effects on the breastfed child or on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with TUKYSA and for 1 week after the last dose.
8.3 Females and Males of Reproductive Potential
TUKYSA can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. TUKYSA is used in combination with trastuzumab and capecitabine. Refer to the Full Prescribing Information of trastuzumab and capecitabine for contraception and infertility information.
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating treatment with TUKYSA.
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with TUKYSA and for 1 week after the last dose.
Males
Advise male patients with female partners of reproductive potential to use effective contraception during treatment with TUKYSA and for 1 week after the last dose.
Infertility
Based on findings from animal studies, TUKYSA may impair male and female fertility [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of TUKYSA in pediatric patients have not been established.
8.5 Geriatric Use
In HER2CLIMB, 82 patients who received TUKYSA were ≥ 65 years, of whom 8 patients were ≥ 75 years. The incidence of serious adverse reactions in those receiving TUKYSA was 34% in patients ≥ 65 years compared to 24% in patients <65 years. The most frequent serious adverse reactions in patients ≥65 years who received TUKYSA were diarrhea (9%), vomiting (6%), and nausea (5%). There were no observed overall differences in the effectiveness of TUKYSA in patients ≥ 65 years compared to younger patients. There were too few patients ≥75 years to assess differences in effectiveness or safety.
In MOUNTAINEER, 12 patients were ≥65 years of age. There were too few patients ≥65 years to assess differences in effectiveness or safety.
8.6 Renal Impairment
The use of TUKYSA in combination with capecitabine and trastuzumab is not recommended in patients with severe renal impairment (creatinine clearance [CLcr]: < 30 mL/min estimated by Cockcroft-Gault Equation), because capecitabine is contraindicated in patients with severe renal impairment. Refer to the Full Prescribing Information of capecitabine for additional information in severe renal impairment.
No dose adjustment is recommended for patients with mild or moderate renal impairment (CLcr: 30 to 89 mL/min).
8.7 Hepatic Impairment
Tucatinib exposure is increased in patients with severe hepatic impairment (Child-Pugh C). Reduce the dose of TUKYSA for patients with severe (Child-Pugh C) hepatic impairment [see Dosage and Administration (2.3), Clinical Pharmacology (12.3)].
No dose adjustment for TUKYSA is required for patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment.
11. Tukysa Description
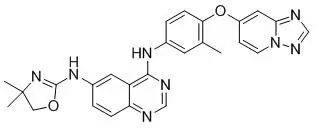
Tucatinib is a kinase inhibitor. The chemical name is (N4-(4-([1,2,4]triazolo[1,5-a]pyridin-7-yloxy)-3-methylphenyl)-N6-(4,4-dimethyl-4,5-dihydrooxazol-2-yl)quinazoline-4,6-diamine. The molecular formula is C26H24N8O2 and the molecular weight is 480.52 g/mol. The chemical structure is as follows:
TUKYSA (tucatinib) is supplied as 50 mg and 150 mg film-coated tablets for oral use and contain the following inactive ingredients:
Tablet core: copovidone, crospovidone, sodium chloride, potassium chloride, sodium bicarbonate, colloidal silicon dioxide, magnesium stearate, and microcrystalline cellulose.
Coating: yellow film coat: polyvinyl alcohol, titanium dioxide, macrogol/polyethylene glycol, talc, and yellow iron oxide non-irradiated.
Each TUKYSA 50 mg tablet contains 10.10 mg (0.258 mEq) potassium and 9.21 mg (0.401 mEq) sodium.
Each TUKYSA 150 mg tablet contains 30.29 mg (0.775 mEq) potassium and 27.64 mg (1.202 mEq) sodium.
12. Tukysa - Clinical Pharmacology
12.1 Mechanism of Action
Tucatinib is a tyrosine kinase inhibitor of HER2. In vitro, tucatinib inhibits phosphorylation of HER2 and HER3, resulting in inhibition of downstream MAPK and AKT signaling and cell proliferation, and showed anti-tumor activity in HER2 expressing tumor cells. In vivo, tucatinib inhibited the growth of HER2 expressing tumors. The combination of tucatinib and trastuzumab showed increased anti-tumor activity in vitro and in vivo compared to either drug alone.
12.2 Pharmacodynamics
Exposure Response Relationship
Tucatinib exposure-response relationships and the time course of pharmacodynamics response have not been fully characterized.
Cardiac Electrophysiology
No large mean increase in QTc (i.e., > 20 ms) was detected following treatment with TUKYSA at the recommended dose of 300 mg taken orally twice daily.
12.3 Pharmacokinetics
Tucatinib AUC0-INF and Cmax increased proportionally over a dosage range from 50 mg to 300 mg (0.17 to 1 times the approved recommended dosage). Time to steady state was approximately 4 days. Steady-state pharmacokinetic parameters following administration of TUKYSA 300 mg twice daily for 7 days in patients with mBC and mCRC are described in Table 9. The geometric mean (CV%) tucatinib AUC accumulation ratios ranged from 2.0 (26) fold to 2.5 (28) fold.
| Tumor Type | AUCss
(ng*h/mL) | Cmax,ss
(ng/mL) | Ctrough,ss
(ng/mL) |
| mBC | 5620 (43) | 747 (45) | 288 (59) |
| mCRC | 3370 (49) | 405 (45) | 197 (63) |
Absorption
The median time to peak plasma concentration of tucatinib was approximately 2 hours (range 1 to 4 hours).
Effects of Food
Following administration of a single oral dose of TUKYSA in 11 subjects after a high-fat meal (approximately 58% fat, 26% carbohydrate, and 16% protein), the mean AUC0-INF increased by 1.5-fold, the Tmax shifted from 1.5 hours to 4 hours, and Cmax was unaltered. The effect of food on the pharmacokinetics of tucatinib was not clinically meaningful.
Distribution
The geometric mean (CV%) apparent volume of distribution of tucatinib at steady-state were 903 (42) L and 829 (21) L in patients with mBC and mCRC, respectively. The plasma protein binding was 97.1% at clinically relevant concentrations.
At steady-state, concentrations of tucatinib and its metabolite ONT-993 in the cerebrospinal fluid were comparable to unbound plasma concentrations.
Elimination
At steady-state, tucatinib effective half-life was approximately 11.9 hours and geometric mean (CV%) apparent clearance was 53 (43) L/h in patients with mBC. Tucatinib effective half-life was approximately 16.4 hours and geometric mean (CV%) apparent clearance was 89 (49) L/h in patients with mCRC.
Metabolism
Tucatinib is metabolized primarily by CYP2C8 and to a lesser extent via CYP3A.
Excretion
Following a single oral dose of 300 mg radiolabeled tucatinib, approximately 86% of the total radiolabeled dose was recovered in feces (16% of the administered dose as unchanged tucatinib) and 4.1% in urine with an overall total recovery of 90% within 13 days post-dose. In plasma, approximately 76% of the plasma radioactivity was unchanged, 19% was attributed to identified metabolites, and approximately 5% was unassigned.
Specific Populations
Age (18-77 years), albumin (19 to 52 g/L), creatinine clearance (CLcr: 60 to 89 mL/min (n = 63); CLcr 30 to 59 mL/min (n = 6)), body weight (41 to 146 kg), sex (male (n = 170), female (n = 113)) and race (White (n = 205), Black (n = 37), or Asian (n = 25)) did not have a clinically meaningful effect on tucatinib exposure.
Renal Impairment
No clinically significant differences in the pharmacokinetics of tucatinib were observed in patients with mild to moderate renal impairment (CLcr: 30 to 89 mL/min by Cockcroft-Gault). The effect of severe renal impairment (CLcr: < 30 mL/min) on the pharmacokinetics of tucatinib is unknown.
Hepatic Impairment
Mild (Child-Pugh A) and moderate (Child-Pugh B) hepatic impairment had no clinically relevant effect on tucatinib exposure. Tucatinib AUC0-INF was increased by 1.6 fold in subjects with severe (Child-Pugh C) hepatic impairment compared to subjects with normal hepatic function.
Drug Interaction Studies
Clinical Studies
| Concomitant Drug (Dose) | TUKYSA Dose | Ratio (90% CI) of Tucatinib Exposure With and Without Concomitant Drug |
|
| Cmax | AUC | ||
| Strong CYP3A Inhibitor Itraconazole (200 mg BID) | 300 mg single dose | 1.3 (1.2, 1.4) | 1.3 (1.3, 1.4) |
| Strong CYP3A/Moderate 2C8 Inducer
Rifampin (600 mg once daily) | 0.6 (0.5, 0.8) | 0.5 (0.4, 0.6) |
|
| Strong CYP2C8 Inhibitor
Gemfibrozil (600 mg BID) | 1.6 (1.5, 1.8) | 3.0 (2.7, 3.5) |
|
| a. Tucatinib reduced the renal clearance of metformin without any effect on glomerular filtration rate (GFR) as measured by iohexol clearance and serum cystatin C. |
|||
| Concomitant Drug (Dose) | TUKYSA Dose | Ratio (90% CI) of Exposure Measures of Concomitant Drug with/without Tucatinib |
|
| Cmax | AUC | ||
| CYP2C8 Substrate
Repaglinide (0.5 mg single dose) | 300 mg single dose | 1.7 (1.4, 2.1) | 1.7 (1.5, 1.9) |
| CYP3A Substrate Midazolam (2 mg single dose) | 3.0 (2.6, 3.4) | 5.7 (5.0, 6.5) |
|
| P-gp Substrate Digoxin (0.5 mg single dose) | 2.4 (1.9, 2.9) | 1.5 (1.3, 1.7) |
|
| MATE1/2-K substratea
Metformin (850 mg single dose) | 1.1 (1.0, 1.2) | 1.4 (1.2, 1.5) |
|
No clinically significant difference in the pharmacokinetics of tucatinib were observed when used concomitantly with omeprazole (proton pump inhibitor) or tolbutamide (sensitive CYP2C9 substrate).
In Vitro Studies
Cytochrome P450 (CYP) Enzymes: Tucatinib is a reversible inhibitor of CYP2C8 and CYP3A and a time-dependent inhibitor of CYP3A, but is not an inhibitor of CYP1A2, CYP2B6, CYP2C9, CYP2C19, or CYP2D6.
Uridine diphosphate (UDP)-glucuronosyl transferase (UGT) Enzymes: Tucatinib is not an inhibitor of UGT1A1.
Transporter Systems: Tucatinib is a substrate of P-gp and BCRP, but is not a substrate of OAT1, OAT3, OCT1, OCT2, OATP1B1, OATP1B3, MATE1, MATE2-K, or BSEP.
Tucatinib inhibits MATE1/MATE2-K-mediated transport of metformin and OCT2/MATE1-mediated transport of creatinine. The observed serum creatinine increase in clinical studies with tucatinib is due to inhibition of tubular secretion of creatinine via OCT2 and MATE1.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with tucatinib.
Tucatinib was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay. Tucatinib was not clastogenic in either an in vitro chromosome aberration assay or an in vivo mouse bone marrow micronucleus assay.
Fertility studies in animals have not been conducted. In repeat-dose toxicity studies up to 13 weeks duration, decreased corpora lutea/corpus luteum cyst, increased interstitial cells of the ovary, atrophy of the uterus, and mucification of the vagina were observed in female rats at doses ≥ 6 mg/kg/day (approximately 0.1 times the human exposure at the recommended dose based on AUC). Atrophy and edema of the testes and oligospermia/germ cell debris in the epididymides were observed in male rats at ≥ 120 mg/kg/day (approximately 13 times the human exposure at the recommended dose based on AUC).
14. Clinical Studies
14.1 HER2-Positive Metastatic Breast Cancer
The efficacy of TUKYSA in combination with trastuzumab and capecitabine was evaluated in 612 patients in HER2CLIMB (NCT02614794), a randomized (2:1), double-blind, placebo-controlled trial. Patients were required to have HER2-positive, unresectable locally advanced or metastatic breast cancer, with or without brain metastases, and prior treatment with trastuzumab, pertuzumab, and ado-trastuzumab emtansine (T-DM1) separately or in combination, in the neoadjuvant, adjuvant or metastatic setting. HER2 positivity was based on archival or fresh tissue tested with an FDA-approved test at a central laboratory prior to enrollment with HER2 positivity defined as HER2 IHC 3+ or ISH positive.
Patients with brain metastases, including those with progressing or untreated lesions, were eligible provided they were neurologically stable and did not require immediate radiation or surgery. The trial excluded patients with leptomeningeal disease. Randomization was stratified by the presence or history of brain metastases (yes vs. no), Eastern Cooperative Oncology Group (ECOG) performance status (0 vs. 1), and region (U.S., Canada, or rest of world).
Patients received TUKYSA 300 mg or placebo orally twice daily with a trastuzumab or a non-US approved trastuzumab product loading dose of 8 mg/kg on Day 1 of Cycle 1 if needed and then a maintenance dose of 6 mg/kg on Day 1 of every 21-day cycle thereafter and capecitabine 1000 mg/m2 orally twice daily on Days 1 through 14 of every 21-day cycle. An alternate trastuzumab dosing regimen was 600 mg administered subcutaneously on Day 1 of every 21-day cycle. Patients were treated until disease progression or unacceptable toxicity. Tumor assessments, including brain-MRI in patients with presence or history of brain metastases at baseline, occurred every 6 weeks for the first 24 weeks and every 9 weeks thereafter.
The major efficacy outcome measure was progression-free survival (PFS) in the first 480 randomized patients assessed by blinded independent central review (BICR) using Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. Additional efficacy outcome measures were evaluated in all randomized patients and included overall survival (OS), PFS among patients with a history or presence of brain metastases (PFSBrainMets), and confirmed objective response rate (ORR).
The median age was 54 years (range: 22 - 82); 116 (19%) patients were age 65 or older. The majority were White (73%) and female (99%) and 51% had an ECOG performance status of 1. Sixty percent had estrogen and/or progesterone receptor-positive disease. Forty-eight percent had a presence or history of brain metastases; of these patients, 23% had untreated brain metastases, 40% had treated but stable brain metastases, and 37% had treated but radiographically progressing brain metastases. Seventy-four percent of patients had visceral metastases. Patients had received a median of 4 (range, 2 to 17) prior lines of systemic therapy and a median of 3 (range, 1 to 14) prior lines of systemic therapy in the metastatic setting. All patients received prior trastuzumab and T-DM1 and all but two patients had prior pertuzumab.
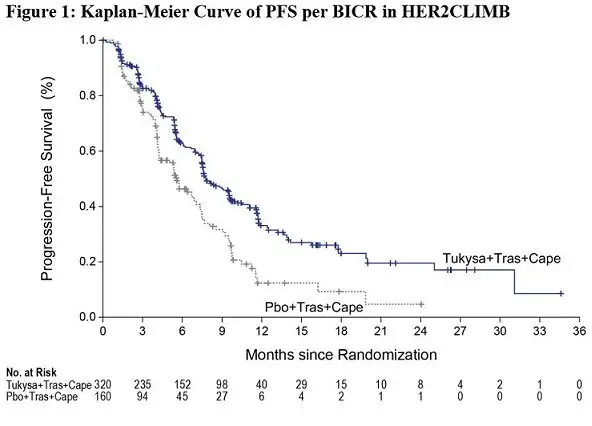
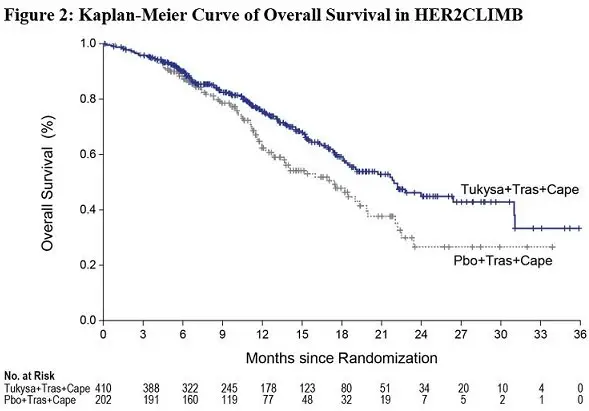
Efficacy results are summarized in Table 12 and Figure 1 and 2. Efficacy results were consistent across patient subgroups defined by stratification factors (presence or history of brain metastases, ECOG status, region of world) and hormone receptor status.
| BICR=blinded independent central review; CI=confidence interval; PFS=progression-free survival; OS=overall survival; ORR=objective response rate; CR=complete response; PR=partial response; DOR=duration of response. 1. Primary PFS analysis conducted in first 480 randomized patients. 2. Hazard ratio and 95% confidence intervals are based on stratified Cox proportional hazards regression model controlling for stratification factors (presence or history of brain metastases, ECOG status, and region of world) 3. Two-sided p-value based on re-randomization procedure (Rosenberger and Lachin 2002) controlling for stratification factors, compared with the allocated alpha of 0.05 4. Two-sided p-value based on re-randomization procedure (Rosenberger and Lachin 2002) controlling for stratification factors, compared with the allocated alpha of 0.0074 for this interim analysis (with 60% of the planned number of events for final analysis) 5. Analysis includes patients with history or presence of parenchymal brain metastases at baseline, including target and non-target lesions. Does not include patients with dural lesions only. 6. Two-sided p-value based on re-randomization procedure (Rosenberger and Lachin 2002) controlling for stratification factors, compared with the allocated alpha of 0.0080 for this interim analysis (with 71% of the planned number of events for final analysis) 7. Two-sided 95% exact confidence interval, computed using the Clopper-Pearson method (1934) 8. Calculated using the complementary log-log transformation method (Collett, 1994) |
|||
| TUKYSA + Trastuzumab + Capecitabine | Placebo + Trastuzumab + Capecitabine |
||
| PFS1 | N=320 | N=160 | |
| Number of events (%) | 178 (56) | 97 (61) | |
| Median, months (95% CI) | 7.8 (7.5, 9.6) | 5.6 (4.2, 7.1) | |
| Hazard ratio (95% CI)2 | 0.54 (0.42, 0.71) | ||
| P-value3 | <0.00001 | ||
| OS | N=410 | N=202 | |
| Number of deaths (%) | 130 (32) | 85 (42) | |
| Median, months (95% CI) | 21.9 (18.3, 31.0) | 17.4 (13.6, 19.9) | |
| Hazard ratio (95% CI)2 | 0.66 (0.50, 0.87) | ||
| P-value4 | 0.00480 | ||
| PFSBrainMets5 | N=198 | N=93 | |
| Number of events (%) | 106 (53.5) | 51 (54.8) | |
| Median, months (95% CI) | 7.6 (6.2, 9.5) | 5.4 (4.1, 5.7) | |
| Hazard ratio (95% CI)2 | 0.48 (0.34, 0.69) | ||
| P-value6 | <0.00001 | ||
| Confirmed ORR for Patients with Measurable Disease | N=340 | N=171 | |
| ORR (95% CI)7 | 40.6 (35.3, 46.0) | 22.8 (16.7, 29.8) | |
| CR (%) | 3 (0.9) | 2 (1.2) | |
| PR (%) | 135 (39.7) | 37 (21.6) | |
| P-value3 | 0.00008 | ||
| DOR | |||
| Median, months (95% CI)8 | 8.3 (6.2, 9.7) | 6.3 (5.8, 8.9) | |
14.2 HER2-Positive Metastatic Colorectal Cancer
The efficacy of TUKYSA in combination with trastuzumab was evaluated in 84 patients in MOUNTAINEER (NCT03043313), an open-label, multicenter trial. Patients were required to have HER2-positive, RAS wild-type, unresectable or metastatic colorectal cancer and prior treatment with fluoropyrimidines, oxaliplatin, irinotecan, and anti-vascular endothelial growth factor (VEGF) monoclonal antibody (mAb). Patients whose disease had deficient mismatch repair (dMMR) proteins or microsatellite instability-high (MSI-H) must have also received an anti-programmed cell death protein-1 (PD-1) mAb. Patients who received prior anti-HER2 targeting therapy were excluded. HER2 positivity as defined by HER2 overexpression or gene amplification was prospectively determined in local laboratories using immunohistochemistry (IHC), in situ hybridization (ISH), and/or next generation sequencing (NGS) on tumor tissue. RAS status was performed as standard of care prior to study entry based on expanded RAS testing including KRAS exons 2, 3, and 4 and NRAS exons 2, 3, and 4.
The median age was 55 years (range: 24 to 77); 12 (14%) patients were age 65 or older. Seventy-seven percent of patients were White, 4% were Black, 4% were Asian, and 4% were Hispanic or Latino. Sixty-one percent of patients were male. Seventy percent of patients had lung metastases, 64% had liver metastases, 60% had an ECOG performance status of 0, 37% had an ECOG performance status of 1, and 4% had an ECOG performance status of 2. Ninety-nine percent of patients received prior treatment with fluoropyrimidine, oxaliplatin, and irinotecan and 83% and 52% received anti-VEGF antibodies and anti-EGFR antibodies respectively; 23%, 38%, and 39% received 1, 2, or ≥3 prior lines of therapy, respectively.
Patients received TUKYSA 300 mg orally twice per day with a loading dose of trastuzumab or a non-US approved trastuzumab product 8 mg/kg intravenously on Day 1 of Cycle 1, followed by a maintenance dose of trastuzumab 6 mg/kg on Day 1 of each subsequent 21-day cycle. Patients were treated until disease progression or unacceptable toxicity.
The major efficacy outcome measures were overall response rate (ORR) and duration of response (DOR) as assessed by blinded independent central review (BICR) according to RECIST version 1.1.
Efficacy results are summarized in Table 13.
| 1. Based on Kaplan-Meier method. 2. Based on observed duration of response. |
|
|
| TUKYSA + trastuzumab N=84 |
| Overall Response Rate (%) (95% CI) | 38 (28, 49) |
| CR (%) | 3 (3.6) |
| PR (%) | 29 (35) |
| Duration of Response | N=32 |
| Median1 , months (95% CI) | 12.4 (8.5, 20.5) |
| Patients with DOR≥6 months2 | 81% |
| Patients with DOR≥12 months2 | 34% |
16. How is Tukysa supplied
16.1 How Supplied
TUKYSA 50 mg tablets are supplied as yellow, film-coated, round tablets containing 50 mg of tucatinib. Each tablet is debossed with “TUC” on one side and “50” on the other side, and is packaged as follows:
50 mg tablets: 60 count in 75 cc bottle: NDC 51144-001-60
TUKYSA 150 mg tablets are supplied as yellow, film-coated, oval-shaped tablets containing 150 mg of tucatinib. Each tablet is debossed with “TUC” on one side and “150” on the other side, and is packaged as follows:
150 mg tablets: 60 count in 75 cc bottle: NDC 51144-002-60
150 mg tablets: 120 count in 150 cc bottle: NDC 51144-002-12
Store at controlled room temperature, 20ºC to 25ºC (68ºF to 77ºF); excursions permitted from 15ºC to 30ºC (59ºF to 86ºF) [see USP Controlled Room Temperature].
Dispense to patient in original container only. Store in original container to protect from moisture. Replace cap securely each time after opening. Do not discard desiccant
Once opened, use within 3 months. Discard any unused tablets 3 months after opening the bottle.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Diarrhea
- Inform patients that TUKYSA has been associated with severe diarrhea. Instruct patients on how to manage diarrhea and to inform their healthcare provider immediately if there is any change in bowel patterns [see Warnings and Precautions (5.1)].
Hepatotoxicity
- Inform patients that TUKYSA has been associated with severe hepatotoxicity and that they should report signs and symptoms of liver dysfunction to their healthcare provider immediately [see Warnings and Precautions (5.2)].
Embryo-Fetal Toxicity
- Inform pregnant women and females of reproductive potential of the risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.3) and Use in Specific Populations (8.1)].
- Advise females of reproductive potential to use effective contraception during treatment with TUKYSA and for 1 week after the last dose [see Use in Specific Populations (8.3)].
- Advise male patients with female partners of reproductive potential to use effective contraception during treatment with TUKYSA and for 1 week after the last dose [see Use in Specific Populations (8.3)].
- Refer to the Full Prescribing Information of trastuzumab and capecitabine for pregnancy and contraception information.
Lactation
- Advise women not to breastfeed during treatment with TUKYSA and for 1 week after the last dose [see Use in Specific Populations (8.2)]. Refer to the Full Prescribing Information of trastuzumab and capecitabine for lactation information.
Infertility
- Advise males and females of reproductive potential that TUKYSA may impair fertility [see Use in Specific Populations (8.3)]. Refer to the Full Prescribing Information of trastuzumab and capecitabine for infertility information.

Manufactured for:
Seagen Inc.
Bothell, WA 98021
1-855-4SEAGEN
TUKYSA, Seagen, and  are US registered trademarks of Seagen Inc.
are US registered trademarks of Seagen Inc.
© 2023 Seagen Inc., Bothell, WA 98021. All rights reserved.
USPI-213411-v04
| This Patient information has been approved by the U.S. Food and Drug Administration Revised: 01/2023 |
|
||
| PATIENT INFORMATION
TUKYSA® (too-KYE-sah) (tucatinib) tablets |
|||
| Important information: If your healthcare provider prescribes TUKYSA in combination with capecitabine for your breast cancer, also read the Patient Information that comes with capecitabine. | |||
| What is TUKYSA?
TUKYSA is a prescription medicine used to treat adults with:
|
|||
| Before taking TUKYSA, tell your healthcare provider about all of your medical conditions, including if you: | |||
|
|||
| Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. TUKYSA may affect the way your other medicines work, and other medicines may affect the way TUKYSA works. Know the medicines you take. Keep a list of all the medicines you take and show it to your healthcare provider and pharmacist every time you get a new medicine.. |
|||
| How should I take TUKYSA? | |||
|
|||
| What are the possible side effects of TUKYSA?
TUKYSA may cause serious side effects, including: |
|||
|
|||
|
|
||
| The most common side effects of TUKYSA in combination with trastuzumab and capecitabine in adults with HER2-positive breast cancer include: | |||
|
|
||
| The most common side effects of TUKYSA in combination with trastuzumab in adults with RAS wild-type HER2-positive colorectal cancer include: | |||
|
|
||
| Your healthcare provider may change your dose of TUKYSA, temporarily stop, or permanently stop treatment with TUKYSA if you have certain side effects.
TUKYSA may cause fertility problems in males and females, which may affect the ability to have children. Talk to your healthcare provider if you have concerns about fertility. These are not all of the possible side effects of TUKYSA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
| How should I store TUKYSA? | |||
|
|||
| Keep TUKYSA and all medicines out of reach of children. | |||
| General information about the safe and effective use of TUKYSA.
Medicines are sometimes prescribed for conditions not listed in the Patient Information. Do not use TUKYSA for a condition for which it was not prescribed. Do not give TUKYSA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about TUKYSA that is written for healthcare professionals. |
|||
| What are the ingredients in TUKYSA?
Active ingredient: tucatinib Inactive ingredients: Tablet core: copovidone, crospovidone, sodium chloride, potassium chloride, sodium bicarbonate, colloidal silicon Tablet coating: yellow film coat: polyvinyl alcohol, titanium dioxide, macrogol/polyethylene glycol, talc, and yellow iron oxide non-irradiated. Manufactured for Seagen Inc., Bothell, WA 98021 TUKYSA is a registered trademark owned by Seagen Inc. ©2023 Seagen Inc. PPI-213411-v04 For more information, call 1-855-473-2436 (1-855-4SEAGEN) or go to www.TUKYSA.com.. |
|||
PRINCIPAL DISPLAY PANEL
NDC 51144-001-60
Rx Only
TUKYSA®
(tucatinib) tablets
50 mg*
Attention: Dispense and store Tukysa
in original container to protect from moisture.
Seagen®
60 Tablets
* Each tablet contains 50 mg tucatinib.
Recommended Dosage: See prescribing information
Store at controlled room temperature,
20°C to 25°C (68° to 77°F); excursions
permitted from 15°C to 30°C (59°F to 86°F)
[see USP Controlled Room Temperature].
KEEP OUT OF REACH OF CHILDREN
Discard unused tablets 3 months after opening.
Mfg for: Seagen Inc.
Bothell, WA 98021 USA
TUKYSA and its logo are US registered
trademarks of Seagen Inc.
©2023 Seagen Inc.
All rights reserved. Printed in USA
00000000
GTIN 00351144001607
LOT: 123456
EXP: MMMYYYY
SN: 12345678901234

NDC 51144-002-60
Rx Only
TUKYSA®
(tucatinib) tablets
150 mg*
Attention: Dispense and store Tukysa
in original container to protect from moisture.
Seagen®
60 Tablets
* Each tablet contains 150 mg tucatinib.
Recommended Dosage: See prescribing information
Store at controlled room temperature,
20°C to 25°C (68° to 77°F); excursions
permitted from 15°C to 30°C (59°F to 86°F)
[see USP Controlled Room Temperature].
KEEP OUT OF REACH OF CHILDREN
Discard unused tablets 3 months after opening.
Mfg for: Seagen Inc.
Bothell, WA 98021 USA
TUKYSA and its logo are US registered
trademarks of Seagen Inc.
©2023 Seagen Inc.
All rights reserved. Printed in USA
00000000
GTIN 00351144002604
LOT: 123456
EXP: MMMYYYY
SN: 12345678901234

NDC 51144-002-12
Rx Only
TUKYSA®
(tucatinib) tablets
150 mg*
Attention: Dispense and store Tukysa
in original container to protect from moisture.
SeaGen®
120 Tablets
* Each tablet contains 150 mg tucatinib.
Recommended Dosage: See prescribing information
Store at controlled room temperature, 20°C to 25°C
(68° to 77°F); excursions permitted from 15°C to 30°C
(59°F to 86°F) [see USP Controlled Room Temperature].
KEEP OUT OF REACH OF CHILDREN
Discard unused tablets 3 months after opening.
Mfg for: Seagen Inc.
Bothell, WA 98021 USA
TUKYSA and its logo are US registered trademarks of Seagen Inc.
©2021 Seagen Inc.
All rights reserved. Printed in USA
1204-02
GTIN 00351144002604
LOT: 123456
EXP: MMMYYYY
SN: 12345678901234

| TUKYSA
tucatinib tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| TUKYSA
tucatinib tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - SEAGEN INC. (028484371) |