Drug Detail:Tyrvaya (Varenicline solution)
Drug Class:
Highlights of Prescribing Information
TYRVAYA™ (varenicline solution) nasal spray
Initial U.S. Approval: 2006
Indications and Usage for Tyrvaya
TYRVAYA (varenicline solution) nasal spray is a cholinergic agonist indicated for the treatment of the signs and symptoms of dry eye disease. (1)
Tyrvaya Dosage and Administration
- •
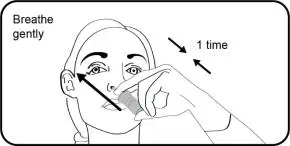
- One spray in each nostril twice daily (approximately 12 hours apart). (2.1)
- •
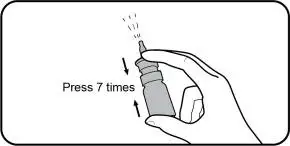
- Prime with seven (7) actuations before initial use. Re-prime with 1 actuation if not used for more than five (5) days. (2.2)
Dosage Forms and Strengths
Nasal spray delivering 0.03 mg of varenicline in each spray (0.05 mL). (3)
Contraindications
None. (4)
Adverse Reactions/Side Effects
The most common adverse reaction reported in 82% of patients was sneezing. Events that were reported in 5-16% of patients were cough, throat irritation, and instillation-site (nose) irritation. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Oyster Point Pharma at 1-877-EYE-0123 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2021
Full Prescribing Information
1. Indications and Usage for Tyrvaya
TYRVAYA (varenicline solution) nasal spray is indicated for the treatment of the signs and symptoms of dry eye disease.
2. Tyrvaya Dosage and Administration
3. Dosage Forms and Strengths
Nasal spray delivering 0.03 mg of varenicline in each spray (0.05 mL).
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In three clinical studies of dry eye disease conducted with varenicline solution nasal spray, 349 patients received at least 1 dose of TYRVAYA. The majority of patients had 31 days of treatment exposure, with maximum exposure of 105 days.
The most common adverse reactions reported in 82% of TYRVAYA treated patients was sneezing. Other common adverse reactions that were reported in >5% of patients include cough (16%), throat irritation (13%), and instillation-site (nose) irritation (8%).
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no available data on TYRVAYA use in pregnant women to inform any drug associated risks. In animal reproduction studies, varenicline did not produce malformations at clinically relevant doses.
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Pregnant rats and rabbits received varenicline succinate during organogenesis at oral doses up to 15 and 30 mg/kg/day, respectively. While no fetal structural abnormalities occurred in either species, maternal toxicity, characterized by reduced body weight gain, and reduced fetal weights occurred in rabbits at the highest dose (4864 times the MRHD on a mg/m2 basis).
In a pre- and postnatal development study, pregnant rats received up to 15 mg/kg/day of oral varenicline succinate from organogenesis through lactation. Maternal toxicity, characterized by a decrease in body weight gain, was observed at 15 mg/kg/day (1216 times the MRHD on a mg/m2 basis). Decreased fertility and increased auditory startle response occurred in offspring at the highest maternal dose of 15 mg/kg/day.
8.2 Lactation
Risk Summary
There are no data on the presence of varenicline in human milk, the effects on the breastfed infant, or the effects on milk production. In animal studies varenicline was present in milk of lactating rats. However, due to species-specific differences in lactation physiology, animal data may not reliably predict drug levels in human milk.
The lack of clinical data during lactation precludes a clear determination of the risk of TYRVAYA to an infant during lactation; however, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for TYRVAYA and any potential adverse effects on the breastfed child from TYRVAYA.
11. Tyrvaya Description
TYRVAYA nasal spray contains varenicline which is a partial nicotinic acetylcholine receptor agonist of α4β2, α4α6β2, α3β4, and α3α5β4 receptors and a full α7 receptor agonist.
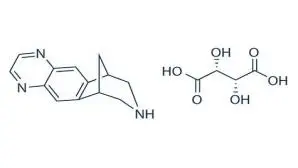
Varenicline, as the tartrate salt, is a powder which is a white to off-white to slightly yellow solid whose chemical name is 7,8,9,10-tetrahydro-6,10-methano-6H-pyrazino[2,3-h][3]benzazepine, (2R,3R)-2,3-dihydroxybutanedioate (1:1). It is highly soluble in water. Varenicline tartrate has a molecular weight of 361.35 Daltons and a molecular formula of C13H13N3 ⋅ C4H6O6. The chemical structure is:
TYRVAYA (varenicline solution) nasal spray is formulated for intranasal use as a clear 0.6 mg/mL strength solution, at pH 6.4. After priming [see Dosage and Administration (2.2)], each actuation delivers a 0.05 mL spray containing 0.03 mg varenicline free base, equivalent to 0.05 mg of varenicline tartrate. The formulation also contains the following inactive ingredients: sodium phosphate dibasic heptahydrate, monobasic sodium phosphate anhydrous, sodium chloride, sodium hydroxide and/or hydrochloric acid (to adjust pH) and water for injection.
12. Tyrvaya - Clinical Pharmacology
12.1 Mechanism of Action
The efficacy of TYRVAYA in dry eye disease is believed to be the result of varenicline's activity at heteromeric sub-type(s) of the nicotinic acetylcholine (nACh) receptor where its binding produces agonist activity and activates the trigeminal parasympathetic pathway resulting in increased production of basal tear film as a treatment for dry eye disease. Varenicline binds with high affinity and selectivity at human α4β2, α4α6β2, α3β4, α3α5β4 and α7 neuronal nicotinic acetylcholine receptors. The exact mechanism of action is unknown at this time.
12.3 Pharmacokinetics
Absorption/Distribution
Following administration of 0.12 mg (0.06 mg per 50-µL spray in each nostril), a strength of varenicline that is higher than the labeled concentration, varenicline can be detected in plasma by 5 minutes, generally achieves peak concentration within 2 hours, with a mean Cmax of 0.34 ng/mL, and has an AUC0-inf of 7.46 h*ng/mL. The systemic exposure (AUC0-inf) following this intranasal dose was approximately 7.5% of the exposure observed following a 1 mg oral dose of varenicline.
Metabolism/Elimination
The mean ± SD elimination half-life of varenicline after intranasal administration is approximately 19 ± 10 hours. Varenicline undergoes minimal metabolism with 92% excreted as unchanged drug in the urine.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Lifetime carcinogenicity studies were performed in CD-1 mice and Sprague-Dawley rats. There was no evidence of a carcinogenic effect in mice administered varenicline by oral gavage for 2 years at doses up to 20 mg/kg/day (810 times the maximum recommended human dose [MRHD], on a mg/m2 basis). Rats were administered varenicline (1, 5, and 15 mg/kg/day) by oral gavage for 2 years. In male rats (n = 65 per sex per dose group), incidences of hibernoma (tumor of the brown fat) were increased at the mid dose (1 tumor, 5 mg/kg/day, 405 times the MRHD on a mg/m2 basis) and maximum dose (2 tumors, 15 mg/kg/day, 1216 times the MRHD on a mg/m2 basis). The clinical relevance of this finding to humans has not been established. There was no evidence of carcinogenicity in female rats.
Mutagenesis
Varenicline was not genotoxic, with or without metabolic activation, in the following assays: Ames bacterial mutation assay; mammalian CHO/HGPRT assay; and tests for cytogenetic aberrations in vivo in rat bone marrow and in vitro in human lymphocytes.
Impairment of Fertility
There was no evidence of impairment of fertility in either male or female Sprague-Dawley rats administered varenicline succinate up to 15 mg/kg/day (1216 times the MRHD on a mg/m2 basis). Maternal toxicity, characterized by a decrease in body weight gain, was observed at 15 mg/kg/day. A decrease in fertility was noted in the offspring of pregnant rats administered varenicline succinate at an oral dose of 15 mg/kg/day. The decrease in fertility in the offspring of treated female rats was not evident at an oral dose of 3 mg/kg/day (243 times the MRHD, on a mg/m2 basis).
14. Clinical Studies
The efficacy of TYRVAYA for the treatment of dry eye disease was supported by two randomized, multi-center, double-masked, vehicle-controlled studies (ONSET-1 and ONSET-2). In the ONSET-1 study, 182 patients were randomized in a 1:1:1:1 ratio to receive one spray in each nostril twice daily of varenicline solution 0.006 mg (N=47), TYRVAYA 0.03 mg (N=48), varenicline solution 0.06 mg (N=44), or vehicle (N=43). In the ONSET-2 study, 758 patients were randomized in a 1:1:1 ratio to receive one spray in each nostril twice daily of TYRVAYA 0.03 mg (N=260), varenicline solution 0.06 mg (N=246), or vehicle (N=252).
The majority of patients were female (74%), the mean (standard deviation [SD]) age was 61 (12.5) years, the mean (SD) baseline anesthetized Schirmer’s score was 5.1 mm (2.9), and the mean (SD) baseline eye dryness score (EDS) was 59.3 (21.6). Use of artificial tears was allowed during the studies. Enrollment criteria included minimal signs [i.e., anesthetized Schirmer's test score (range, 0-10 mm) and corneal fluorescein staining (range, 2-14)] and was not limited by baseline EDS (range, 2-100).
Efficacy
Tear film production was measured by anesthetized Schirmer’s score assessed using a Schirmer’s strip (0-35 mm). The average baseline Schirmer’s score was 5.0 mm in the ONSET-1 study and 5.1 mm in the ONSET-2 study. Of the patients treated with TYRVAYA, 52% achieved ≥10 mm increase in Schirmer’s score from baseline in the ONSET-1 study and 47% achieved ≥10 mm increase in Schirmer’s score from baseline in the ONSET-2 study, compared to 14% and 28% of vehicle-treated patients in the ONSET-1 study and the ONSET-2 study, respectively at Day 28 (see Table 1). Of the patients treated with TYRVAYA, the mean change in Schirmer’s score was 11.7 mm and 11.3 mm as compared to 3.2 mm and 6.3 mm in the vehicle treated patients in the ONSET-1 study and ONSET-2 study, respectively at Day 28.
|
ONSET-1 |
ONSET-2 |
|||
|
TYRVAYA
|
Vehicle
|
TYRVAYA
|
Vehicle
|
|
|
≥ 10-mm increase in tear production (% of eyes) at Day 28 |
52% |
14% |
47% |
28% |
|
Proportion Difference (95% CI) |
38% (21%, 56%) |
20% (11%, 28%) |
||
|
p-value versus control |
<0.01 |
<0.01 |
||
Cochran-Mantel-Haenszel (CMH) test controlling for study site, baseline Schirmer’s test score (STS), and baseline EDS.
All randomized and treated patients were included in the analysis and missing data were imputed using last-available data.
16. How is Tyrvaya supplied
16.1 How Supplied
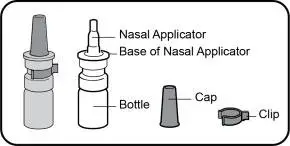
TYRVAYA (varenicline solution) nasal spray is available in a carton containing two (2) nasal spray amber glass Type I bottles. Each bottle consists of a white nasal pump and blue dust cover, delivering 0.03 mg varenicline per spray (0.05 mL). Each bottle delivers one spray in each nostril twice daily for 15 days.
Two nasal spray bottles in each carton, containing 60 sprays per bottle, equivalent to 30-days’ supply with one spray in each nostril twice daily (NDC 73521-030-02).
17. Patient Counseling Information
- •
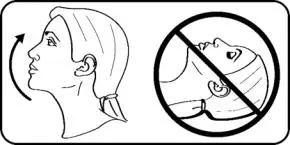
- Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- •
- Instruct patients that TYRVAYA works to increase tear production in the eye after being sprayed in the nose.
- •
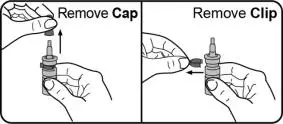
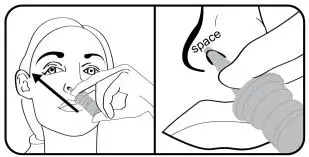
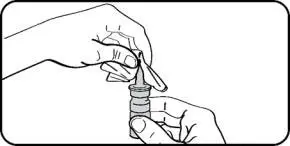
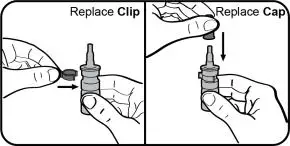
- Instruct patients to prime the bottle before using it for the first time by pumping seven (7) sprays into the air away from the face and to re-prime it by pumping 1 spray into the air away from the face if the bottle has not been used in more than five (5) days.
- •
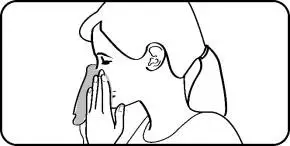
- Instruct patients to wipe the nasal applicator with a clean tissue after each use.
- •
- Instruct patients to not shake or freeze the bottle.
Manufactured for: Oyster Point Pharma, Inc, 202 Carnegie Center, Suite 109, Princeton, NJ 08540
Copyrights and Trademarks are property of their respective owners.
TYRVAYA™ is a trademark of Oyster Point Pharma, Inc.
TYRVAYA™ and/or the use of TYRVAYA™ in a method may be covered by one or more patents or patent applications, available at www.oysterpointrx.com/patent-notices.
©2021 Oyster Point Pharma, Inc. All Rights Reserved.
Issued: Oct/2021
|
Patient Information
|
|
What is TYRVAYA?
|
|
Before you use TYRVAYA, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. |
|
How should I use TYRVAYA?
|
|
What are the possible side effects of TYRVAYA?
|
|
How should I store TYRVAYA?
Keep TYRVAYA and all medicines out of the reach of children. |
|
General information about the safe and effective use of TYRVAYA.
|
|
What are the ingredients in TYRVAYA?
|
- This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 10/2021
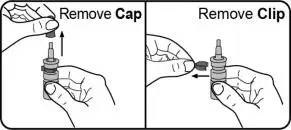
Instructions for Use
Tyrvaya™ (Teer-vye-ah)
(varenicline solution)
nasal spray, for intranasal use
Read this Instructions for Use before you start using TYRVAYA and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
- •
-
Reprime: If you do not use TYRVAYA for more than 5 days, you will need to reprime the nasal spray bottle with 1 spray before you start using it. To reprime, hold the nasal spray bottle upright and away from your face and press and release the nasal spray applicator 1 time.
- •
- Avoid priming the nasal spray bottle more than needed: Priming the nasal spray bottle more than needed will reduce the amount of medicine in the nasal spray bottle.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
TYRVAYA™ is a trademark of Oyster Point Pharma, Inc.
TYRVAYA™ and/or the use of TYRVAYA™ in a method may be covered by one or more patents or patent applications, available at www.oysterpointrx.com/patent-notices.
Manufactured for: Oyster Point Pharma, Inc., 202 Carnegie Center, Suite 109, Princeton, NJ 08540
- ©2021 Oyster Point Pharma, Inc. Issued: 10/2021
PRINCIPAL DISPLAY PANEL
NDC 73521-030-02
tyrvaya
(varenicline) nasal spray
0.03 mg per spray
2 nasal spray bottles
for a 30-day supply
PRINCIPAL DISPLAY PANEL
NDC 73521-030-01
tyrvaya
(varenicline) nasal spray
0.03 mg per spray
Contains 60 sprays (Net Content 4.2 mL)
| TYRVAYA
varenicline spray |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Oyster Point Pharma (080478750) |