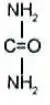Drug Detail:Ure-k (Urea topical [ yoo-ree-a-top-ik-al ])
Drug Class: Topical emollients
Precautions
After applying this medication, wash hands and unaffected areas thoroughly. Stop use and ask a doctor if redness or irritation develops. If swallowed, get medical help or contact Poison Control Center right away. KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN.
| URE-K
urea cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Solutech Pharmaceuticals LLC (080040396) |