Drug Detail:Ventavis (Iloprost inhalation [ ill-o-prost ])
Drug Class: Agents for pulmonary hypertension
Highlights of Prescribing Information
VENTAVIS® (iloprost) inhalation solution, for oral inhalation use
Initial U.S. Approval: 2004
Indications and Usage for Ventavis
VENTAVIS is a prostacyclin mimetic indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) to improve a composite endpoint consisting of exercise tolerance, symptoms (NYHA Class), and lack of deterioration. Studies establishing effectiveness included predominately patients with NYHA Functional Class III-IV symptoms and etiologies of idiopathic or heritable PAH (65%) or PAH associated with connective tissue diseases (23%). (1.1).
Ventavis Dosage and Administration
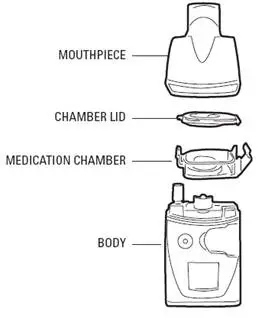
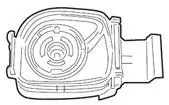
VENTAVIS is intended to be inhaled using the I-neb® AAD® System. Patients should receive 6 to 9 doses (inhalations) per day (minimum of 2 hours between doses during waking hours) as follows:
- Starting dose: 2.5 mcg (2.1).
- Uptitrate to 5 mcg if 2.5 mcg is well tolerated (2.1).
- Maintenance dose: 5 mcg (2.1).
| Delivered dose from ampule of: | ||
|---|---|---|
| Nebulizer | 10 mcg/mL | 20 mcg/mL |
| I-neb® AAD® | 2.5 or 5 mcg from one ampule | 5 mcg from one ampule |
- The 20 mcg/mL concentration is for patients who repeatedly experience extended treatment times (2.1).
Dosage Forms and Strengths
1 mL ampules in two concentrations: 10 mcg/mL and 20 mcg/mL (3).
Contraindications
None (4).
Warnings and Precautions
- Hypotension leading to syncope has been observed. Monitor vital signs while initiating VENTAVIS. VENTAVIS should not be administered in patients with systolic blood pressure below 85 mmHg (5.1).
- Pulmonary venous hypertension: Discontinue if pulmonary edema is present (5.2).
- May cause bronchospasm: Patients with a history of hyperreactive airway disease may be more sensitive (5.3).
Adverse Reactions/Side Effects
Most common (≥3% placebo adjusted) adverse reactions are vasodilation (flushing), cough increased, headache, trismus, insomnia, nausea, hypotension, vomiting, alkaline phosphatase increased, flu syndrome, back pain, tongue pain, palpitations, syncope, GGT increased, muscle cramps, hemoptysis, and pneumonia (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Janssen at 1-800-526-7736 (1-800-JANSSEN) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- VENTAVIS has the potential to increase the hypotensive effect of vasodilators and antihypertensive agents (7.1).
- There is a potential for increased risk of bleeding, particularly in patients maintained on anticoagulants (7.2).
Use In Specific Populations
- Lactation: Advise not to breastfeed (8.2).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2022
Related/similar drugs
sildenafil, tadalafil, Adcirca, Revatio, Opsumit, ambrisentanFull Prescribing Information
1. Indications and Usage for Ventavis
1.1 Pulmonary Arterial Hypertension
VENTAVIS is indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) to improve a composite endpoint consisting of exercise tolerance, symptoms (NYHA Class), and lack of deterioration. Studies establishing effectiveness included predominately patients with NYHA Functional Class III–IV symptoms and etiologies of idiopathic or heritable PAH (65%) or PAH associated with connective tissue diseases (23%) [see Clinical Studies (14)].
2. Ventavis Dosage and Administration
2.1 Recommended Dosing
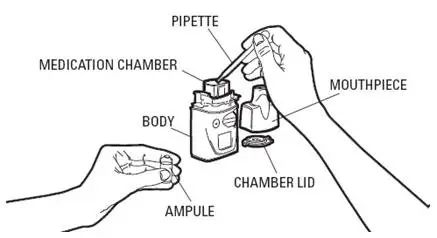
VENTAVIS is intended to be inhaled using the I-neb® AAD® System. The recommended initial inhaled dose is 2.5 mcg (as delivered at the mouthpiece). If well tolerated, increase dosing to 5.0 mcg and maintain at that dose; otherwise maintain the dose at 2.5 mcg [see Warnings and Precautions (5.1)]. VENTAVIS should be taken 6 to 9 times per day (no more than once every 2 hours) during waking hours, according to individual need and tolerability. The maximum daily dose evaluated in clinical studies was 45 mcg (5 mcg 9 times per day).
Direct mixing of VENTAVIS with other medications in the I-neb® AAD® System has not been evaluated; do not mix with other medications. To avoid potential interruptions in drug delivery due to equipment malfunctions, the patient should have easy access to a back-up I-neb®AAD® System.
For each inhalation session, only a mouthpiece should be used. Avoid skin or eye contact with VENTAVIS solution. Do not orally ingest VENTAVIS solution.
VENTAVIS is supplied in 1 mL ampules in two concentrations: 10 mcg/mL and 20 mcg/mL.
| Delivered dose from ampule of: | ||
|---|---|---|
| Nebulizer | 10 mcg/mL | 20 mcg/mL |
| I-neb® AAD® | 2.5 or 5 mcg from one ampule | 5 mcg from one ampule |
The 20 mcg/mL concentration is intended for patients who are maintained at the 5 mcg dose and who have repeatedly experienced extended treatment times which could result in incomplete dosing. Transitioning patients to the 20 mcg/mL concentration using the I-neb® AAD® System will decrease treatment times to help maintain patient compliance.
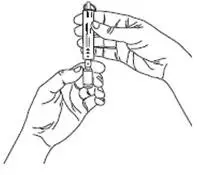
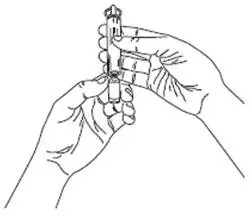
For each inhalation session, the entire contents of each opened ampule of VENTAVIS should be transferred into the I-neb® AAD® System medication chamber immediately before use. Discard any solution remaining in the medication chamber after each inhalation session. Patients should follow the manufacturer's instructions for cleaning the I-neb® AAD® System components after each dose administration.
5. Warnings and Precautions
5.1 Risk of Syncope
Monitor vital signs while initiating VENTAVIS. Do not initiate VENTAVIS in patients with systolic blood pressure below 85 mmHg. Syncope can also occur in association with pulmonary arterial hypertension, particularly in association with physical exertion. The occurrence of exertional syncope may reflect a therapeutic gap or insufficient efficacy, and the need to adjust dose or change therapy should be considered.
5.2 Pulmonary Venous Hypertension
Should signs of pulmonary edema occur when inhaled VENTAVIS is administered in patients with pulmonary hypertension, stop treatment immediately, as this may be a sign of pulmonary venous hypertension.
5.3 Bronchospasm
VENTAVIS inhalation can induce bronchospasm. Bronchospasm may be more severe or frequent in patients with a history of hyperreactive airways. VENTAVIS has not been evaluated in patients with chronic obstructive pulmonary disease (COPD), severe asthma, or with acute pulmonary infections.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Pre-marketing safety data on VENTAVIS were obtained from 215 patients with PAH receiving iloprost in two 12-week clinical trials and two long-term extensions. Patients received inhaled VENTAVIS for periods of from 1 day to more than 3 years. The median number of weeks of exposure was 15. Forty patients completed 12 months of open-label treatment with iloprost.
The following table shows adverse events reported by at least 4 VENTAVIS patients and reported at least 3% more frequently for VENTAVIS patients than placebo patients in the 12-week placebo-controlled study.
| Adverse Event | VENTAVIS N=101 | Placebo N=102 | Placebo subtracted % |
|---|---|---|---|
| Vasodilation (flushing) | 27 | 9 | 18 |
| Cough increased | 39 | 26 | 13 |
| Headache | 30 | 20 | 10 |
| Trismus | 12 | 3 | 9 |
| Insomnia | 8 | 2 | 6 |
| Nausea | 13 | 8 | 5 |
| Hypotension | 11 | 6 | 5 |
| Vomiting | 7 | 2 | 5 |
| Alk phos increased | 6 | 1 | 5 |
| Flu syndrome | 14 | 10 | 4 |
| Back pain | 7 | 3 | 4 |
| Tongue pain | 4 | 0 | 4 |
| Palpitations | 7 | 4 | 3 |
| Syncope | 8 | 5 | 3 |
| GGT increased | 6 | 3 | 3 |
| Muscle cramps | 6 | 3 | 3 |
| Hemoptysis | 5 | 2 | 3 |
| Pneumonia | 4 | 1 | 3 |
Pre-marketing serious adverse events reported with the use of inhaled VENTAVIS and not shown in Table 1 include congestive heart failure, chest pain, supraventricular tachycardia, dyspnea, peripheral edema, and kidney failure.
In a small clinical trial (the STEP trial) [see Clinical Studies (14)], safety trends in patients receiving concomitant bosentan and VENTAVIS were consistent with those observed in the larger experience of the Phase 3 study in patients receiving only VENTAVIS or bosentan.
6.2 Postmarketing Experience
The following adverse reactions have been identified during the post-approval use of VENTAVIS. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cases of bronchospasm and wheezing have been reported, particularly in patients with a history of hyperreactive airways [see Warnings and Precautions (5.3)]. Bleeding events most commonly reported as epistaxis and hemoptysis were observed on VENTAVIS treatment [see Drug Interactions (7.2)]. Cases of thrombocytopenia, dizziness, diarrhea, mouth and tongue irritation, nasal congestion, dysgeusia, hypersensitivity, and rash have also been reported with the use of VENTAVIS.
7. Drug Interactions
7.1 Antihypertensives and Vasodilators
In studies in normal subjects, there was no pharmacodynamic interaction between intravenous iloprost and either nifedipine, diltiazem, or captopril. However, VENTAVIS has the potential to increase the hypotensive effect of vasodilators and antihypertensive agents.
8. Use In Specific Populations
8.5 Geriatric Use
Clinical studies of VENTAVIS did not include sufficient numbers of subjects aged 65 and older to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
VENTAVIS has not been evaluated in subjects with impaired hepatic function. However, in an intravenous iloprost study in patients with liver cirrhosis, the mean clearance in Child- Pugh Class B subjects (n=5) was approximately 10 mL/min/kg (half that of healthy subjects). Following oral administration, the mean AUC0–8h in Child-Pugh Class B subjects (n=3) was 1725 pg*h/mL compared to 117 pg*h/mL in normal subjects (n=4) receiving the same oral iloprost dose. In Child-Pugh Class A subjects (n=5), the mean AUC0–8h was 639 pg*h/mL. Although exposure increased with hepatic impairment, there was no effect on half-life.
8.7 Renal Impairment
VENTAVIS has not been evaluated in subjects with impaired renal function. However, in a study with intravenous infusion of iloprost in patients with end-stage renal failure, patients requiring intermittent dialysis treatment (n=7) had a mean AUC0–4h of 230 pg*h/mL compared to 54 pg*h/mL in patients with renal failure (n=8) not requiring intermittent dialysis and 48 pg*h/mL in normals. The half-life was similar in both groups. The effect of dialysis on iloprost exposure has not been evaluated.
10. Overdosage
Cases of overdose have been reported. Frequently observed symptoms following overdose are dizziness, headache, flushing, nausea, jaw pain or back pain. Hypotension, vomiting, and diarrhea are possible. A specific antidote is not known. Interruption of the inhalation session, monitoring, and symptomatic measures are recommended.
11. Ventavis Description
VENTAVIS® (iloprost) Inhalation Solution is a clear, colorless, sterile solution containing iloprost formulated for inhalation via the I-neb® AAD® (Adaptive Aerosol Delivery) System. VENTAVIS is supplied in 1 mL single-use glass ampules containing either 10 mcg/mL or 20 mcg/mL.
For the 10 mcg/mL solution, one mL of the solution contains 0.01 mg iloprost and also contains 0.81 mg ethanol, approximately 0.51 mg hydrochloric acid (for pH adjustment to 8.1) in water for injection, 9.0 mg sodium chloride, and 0.121 mg tromethamine.
For the 20 mcg/mL solution, one mL of the solution contains 0.02 mg iloprost and also contains 1.62 mg ethanol, approximately 0.76 mg hydrochloric acid (for pH adjustment to 8.4) in water for injection, 9.0 mg sodium chloride, and 0.242 mg tromethamine.
The solution contains no preservatives.
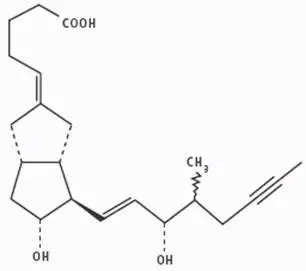
The chemical name for iloprost is (E)-(3aS, 4R, 5R, 6aS)-hexahydro-5-hydroxy-4-[(E)-(3S,4RS)-3-hydroxy-4-methyl-1-octen-6-ynyl]-Δ2(1H),Δ-pentalenevaleric acid. Iloprost consists of a mixture of the 4R and 4S diastereoisomers at a ratio of approximately 53:47. Iloprost is an oily substance, which is soluble in methanol, ethanol, ethyl acetate, acetone, and pH 7 buffer, sparingly soluble in buffer pH 9, and very slightly soluble in distilled water, buffer pH 3, and buffer pH 5. The molecular formula of iloprost is C22H32O4. Its relative molecular weight is 360.49. The structural formula is shown below:
12. Ventavis - Clinical Pharmacology
12.1 Mechanism of Action
Iloprost is a synthetic analog of prostacyclin PGI2. Iloprost dilates systemic and pulmonary arterial vascular beds. It also affects platelet aggregation but the relevance of this effect to the treatment of pulmonary hypertension is unknown. The two diastereoisomers of iloprost differ in their potency in dilating blood vessels, with the 4S isomer substantially more potent than the 4R isomer.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Iloprost was not mutagenic in bacterial and mammalian cells in the presence or absence of extrinsic metabolic activation. Iloprost did not cause chromosomal aberrations in vitro in human lymphocytes and was not clastogenic in vivo in NMRI/SPF mice. There was no evidence of a tumorigenic effect of iloprost clathrate (13% iloprost by weight) in Sprague-Dawley rats dosed orally for up to 8 months at doses of up to 125 mg/kg/day (Cmax of 45 ng/mL serum), followed by 16 months at 100 mg/kg/day, or in Crl:CD-1®(ICR)BR albino mice dosed orally for up to 24 months at doses of up to 125 mg/kg/day (Cmax of 156 ng/mL serum). The recommended clinical dosage regimen for iloprost (5 mcg) affords a serum Cmax of 0.16 ng/mL. Fertility of males or females was not impaired in Han-Wistar rats at intravenous doses up to 1 mg/kg/day.
14. Clinical Studies
A randomized, double-blind, multi-center, placebo-controlled trial was conducted in 203 adult patients (inhaled iloprost: n=101; placebo: n=102) with NYHA Class III or IV PAH (WHO Group 1); idiopathic in 53%, associated with connective tissue disease, including CREST and scleroderma, in 17%, or associated with anorexigen use in 2%) or PAH related to chronic thromboembolic disease (WHO Group 4; 28%). Inhaled iloprost (or placebo) was added to patients' current therapy, which could have included anticoagulants, vasodilators (e.g., calcium channel blockers), diuretics, oxygen, and digoxin, but not PGI2 (prostacyclin or its analogs) or endothelin receptor antagonists. Patients received 2.5 or 5.0 mcg of iloprost by repeated inhalations 6 to 9 times per day during waking hours. The mean age of the entire study population was 52 years and 68% of the patients were female. The majority of patients (59%) were NYHA Class III. The baseline 6-minute walk test values reflected a moderate exercise limitation (the mean was 332 meters for the iloprost group and 315 meters for the placebo group). In the iloprost group, the median daily inhaled dose was 30 mcg (range of 12.5 to 45 mcg/day). The mean number of inhalations per day was 7.3. Ninety percent of patients in the iloprost group never inhaled study medication during the nighttime.
The primary efficacy endpoint was clinical response at 12 weeks, a composite endpoint defined by: a) improvement in exercise ability (6-minute walk test) by at least 10% versus baseline evaluated 30 minutes after dosing, b) improvement by at least one NYHA class versus baseline, and c) no death or deterioration of pulmonary hypertension. Deterioration required two or more of the following criteria: 1) refractory systolic blood pressure < 85 mmHg, 2) worsening of right heart failure with cardiac edema, ascites, or pleural effusion despite adequate background therapy, 3) rapidly progressive cardiogenic hepatic failure (e.g., leading to an increase of GOT or GPT to >100 U/L, or total bilirubin ≥5 mg/dL), 4) rapidly progressive cardiogenic renal failure (e.g., decrease of estimated creatinine clearance to ≤50% of baseline), 5) decrease in 6-minute walking distance by ≥30% of baseline value, 6) new long-term need for i.v. catecholamines or diuretics, 7) cardiac index ≤1.3 L/min/m2, 8) CVP ≥22 mmHg despite adequate diuretic therapy, and 9) SVO2 ≤45% despite nasal O2 therapy.
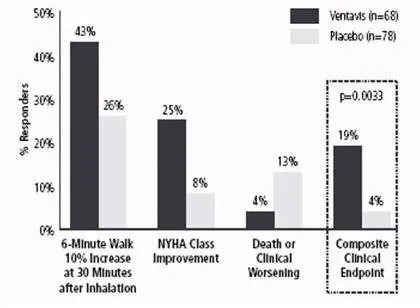
Although effectiveness was seen in the full population (response rates for the primary composite endpoint of 17% and 5%; p=0.007), there was inadequate evidence of benefit in patients with pulmonary hypertension associated with chronic thromboembolic disease (WHO Group 4); the results presented are therefore those related to patients with PAH (WHO Group 1). The response rate for the primary efficacy endpoint among PAH patients was 19% for the iloprost group, compared with 4% for the placebo group (p=0.0033). All three components of the composite endpoint favored iloprost (Figure 1).
Figure 1: Composite Primary Endpoint for PAH Patients (WHO Group 1)
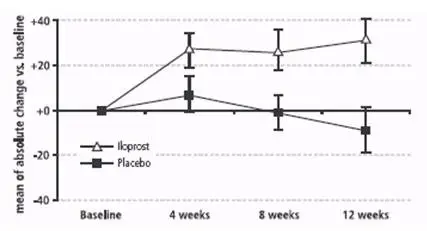
The absolute change in 6-minute walk distance (Figure 2) measured (using all available data and no imputation) 30 minutes after inhalation among patients with PAH was greater in the iloprost group compared to the placebo group at all time points. At Week 12, the placebo-corrected difference was 40 meters (p < 0.01). When walk distance was measured immediately prior to inhalation, the improvement compared to placebo was approximately 60% of the effect seen at 30 minutes after inhalation.
Figure 2: Change (Mean ± SEM) in 6-Minute Walk Distance 30 Minutes Post-inhalation in PAH Patients (WHO Group 1).
The effect of VENTAVIS in various subgroups is shown in Table 2.
| Composite Clinical Endpoint | 6-Minute Walk (m)* | |||||||
|---|---|---|---|---|---|---|---|---|
| n | VENTAVIS n (%) | n | Placebo n (%) | n | VENTAVIS (mean ±SD) | n | Placebo (mean ±SD) |
|
|
||||||||
| All Subjects with PAH†
| 68 | 13 (19%) | 78 | 3 (4%) | 64 | 31 ± 76 | 65 | -9 ± 79 |
| NYHA III | 40 | 7 (18%) | 47 | 2 (4%) | 39 | 24 ± 72 | 43 | -16 ± 86 |
| NYHA IV | 28 | 6 (21%) | 31 | 1 (3%) | 25 | 43 ± 82 | 22 | 6 ± 63 |
| Male | 23 | 5 (22%) | 24 | 0 (0%) | 21 | 37 ± 81 | 21 | -22 ± 77 |
| Female | 45 | 8 (18%) | 54 | 3 (6%) | 43 | 29 ± 74 | 44 | -2 ± 81 |
| Age ≤55 | 41 | 6 (15%) | 40 | 2 (5%) | 39 | 24 ± 79 | 32 | -5 ± 78 |
| Age >55 | 27 | 7 (26%) | 38 | 1 (3%) | 25 | 42 ± 71 | 33 | -13 ± 81 |
Hemodynamic assessments obtained at Week 12 before inhalation in both groups (at least 2 hours after a previous dose, trough) and after inhalation in the iloprost group (approximately 15 minutes after a dose, peak), are shown in Table 3. The relationship between hemodynamic changes and clinical effects is unknown.
| Baseline | Mean (± SD) change from baseline at Week 12 | ||||
|---|---|---|---|---|---|
| Parameter | Iloprost | Placebo | Iloprost | Placebo | |
| Before Inhalation | After Inhalation | ||||
|
|||||
| PVR (dyn∙s∙cm–5) | 1029 ± 390 | 1041 ± 493 | –9 ± 275 (n=76) | –239 ± 279 (n=70) | +96 ± 323 (n=77) |
| mPAP (mmHg) | 53 ± 12 | 54 ± 14 | –0.2 ± 7.3 (n=93) | –4.6 ± 9.3 (n=90) | –0.1 ± 6.9 (n=82) |
| CO (L/min) | 3.8 ± 1.1 | 3.8 ± 0.9 | +0.1 ± 0.9 (n=91) | +0.5 ± 1.1 (n=89) | –0.2 ± 0.8 (n=80) |
| SVO2 (%) | 60 ± 8 | 60± 8 | –1.1 ± 7.6 (n=72) | +1.8 ± 8.3 (n=70) | –3.2 ± 6.7 (n=63) |
In a small, randomized, double-blind, placebo-controlled study (the STEP trial), 34 patients treated with bosentan 125 mg bid for at least 16 weeks tolerated the addition of inhaled iloprost (up to 5 mcg 6 to 9 times per day during waking hours). The mean daily inhaled dose was 27 mcg and the mean number of inhalations per day was 5.6.
16. How is Ventavis supplied
VENTAVIS® (iloprost) Inhalation Solution is supplied in cartons of 30×1 mL clear glass single-use ampules as follows:
1 mL ampule containing iloprost 10 mcg per mL (NDC 66215-302-00), carton of 30 (NDC 66215-302-30)
1 mL ampule containing iloprost 20 mcg per mL (NDC 66215-303-00), carton of 30 (NDC 66215-303-30)
17. Patient Counseling Information
Advise patients to read the FDA-approved patient labeling (Patient Information).
Advise patients that they may have a fall in blood pressure with VENTAVIS, so they may become dizzy or even faint. They should stand up slowly when they get out of a chair or bed. If fainting gets worse, patients should consult their physicians about dose adjustment.
Advise patients that VENTAVIS should be inhaled at intervals of not less than 2 hours and that the acute benefits of VENTAVIS may not last 2 hours. Thus, patients may want to adjust times of administration to cover planned activities.
| VENTAVIS
iloprost solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| VENTAVIS
iloprost solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Actelion Pharmaceuticals US, Inc. (002641228) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BERLIMED SA | 560874307 | MANUFACTURE(66215-302, 66215-303) , LABEL(66215-302, 66215-303) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bayer AG | 315097875 | API MANUFACTURE(66215-302, 66215-303) , ANALYSIS(66215-302, 66215-303) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bayer AG | 323208116 | API MANUFACTURE(66215-302, 66215-303) , ANALYSIS(66215-302, 66215-303) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Currenta GmbH & Co. OHG | 331575303 | ANALYSIS(66215-302, 66215-303) | |