Drug Detail:Viracept (Nelfinavir [ nel-fin-a-veer ])
Drug Class: Protease inhibitors
Highlights of Prescribing Information
VIRACEPT® (nelfinavir mesylate) Tablets, for oral use
VIRACEPT® (nelfinavir mesylate) Oral Powder, for oral use
Initial U.S. Approval: 1997
Indications and Usage for Viracept
VIRACEPT is a protease inhibitor indicated for the treatment of HIV-1 infection in combination with other antiretroviral agents. (1)
Viracept Dosage and Administration
- See full prescribing information for administration instructions (2)
- Adults and adolescents 13 years and older (tablets): 1250 mg twice daily or 750 mg three times daily with a meal (2.1)
- Children 2 to less than 13 years (oral powder or 250 mg tablets): 45 to 55 mg/kg twice daily or 25 to 35 mg/kg three times daily with a meal. Refer to Tables 1 and 2 of the full prescribing information for specific dosing guidelines based on age and body weight (2.2)
Dosage Forms and Strengths
- Tablet: 250 mg, 625 mg nelfinavir free base (3)
- Oral Powder: 50 mg/g nelfinavir free base (3)
Contraindications
Coadministration with drugs that are highly dependent on CYP3A for clearance and which elevated concentrations are associated with serious and/or life-threatening events (4)
Warnings and Precautions
ALERT: Find out about medicines that should not be taken with VIRACEPT.
- The concomitant use of VIRACEPT and certain other drugs may result in known or potentially significant drug interactions. Consult the full prescribing information prior to and during treatment for potential drug interactions (5.1, 7.3)
- Hepatic impairment: should not be used in patients with either moderate or severe hepatic impairment (2.4, 5.2)
- Phenylketonuria: the oral powder contains 11.2 mg phenylalanine per gram of powder (5.3)
- Diabetes mellitus/hyperglycemia: new onset or exacerbation of pre-existing diabetes mellitus and hyperglycemia reported with protease inhibitors. In some cases after treatment discontinuation, hyperglycemia persisted (5.4)
- Hemophilia: increased bleeding, including spontaneous skin hematomas and hemarthrosis reported with protease inhibitors. In more than half of the cases, protease inhibitors was continued or reintroduced (5.5)
- Fat redistribution: observed with antiretroviral therapy (5.6)
- Immune reconstitution syndrome: reported with combination antiretroviral therapy, including VIRACEPT. Patients may develop an inflammatory response to indolent or residual opportunistic infections (5.7)
Adverse Reactions/Side Effects
- Most common adverse reactions (≥2%) of moderate or severe intensity in adults and adolescents (13 years and older) are diarrhea, nausea, rash, and flatulence (6.1).
- Most common adverse reactions in pediatric patients (2 to less than 13 years) are diarrhea, leukopenia/neutropenia, rash, anorexia, and abdominal pain. (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Co-administration of VIRACEPT with other drugs (CYP3A substrates) can alter the concentration of these other drugs, and other drugs (inhibitors and/or inducers of CYP3A or CYP2C19) may alter the concentrations of nelfinavir. The potential drug-drug concentrations must be considered prior to and during therapy (4, 7, 12.3)
- VIRACEPT should be given with food one hour after or more than 2 hours before didanosine (7)
Use In Specific Populations
- Use during pregnancy if the potential benefit justifies the potential risk to the fetus (8.1)
- Lactation: Patients infected with HIV should be instructed not to breastfeed due to the potential for HIV transmission (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2021
Full Prescribing Information
1. Indications and Usage for Viracept
VIRACEPT in combination with other antiretroviral agents is indicated for the treatment of HIV-1 infection.
2. Viracept Dosage and Administration
2.1 Adults and Adolescents (13 years and older)
The recommended dose is 1250 mg (five 250 mg tablets or two 625 mg tablets) twice daily or 750 mg (three 250 mg tablets) three times daily. VIRACEPT should be taken with a meal. Patients unable to swallow the 250 or 625 mg tablets may dissolve the tablets in a small amount of water [see Dosage and Administration (2.3)].
2.2 Pediatric Patients (2 to less than 13 years)
In children 2 years of age and older, the recommended oral dose of VIRACEPT Oral Powder or 250 mg tablets is 45 to 55 mg/kg twice daily or 25 to 35 mg/kg three times daily. All doses should be taken with a meal. Doses higher than the adult maximum dose of 2500 mg per day have not been studied in children.
For children unable to swallow tablets, VIRACEPT 250 mg tablet(s) may be dissolved in a small amount of water or, VIRACEPT Oral Powder may be administered [see Dosage and Administration (2.3)].
The healthcare provider should assess appropriate formulation and dosage for each patient. Tables 1 and 2 provide dosing guidelines for VIRACEPT tablets and powder based on age and body weight.
| Body weight | Twice daily (BID) 45 – 55 mg/kg ≥2 years | Three times daily (TID) 25 – 35 mg/kg ≥2 years |
|---|---|---|
| Number of tablets (250 mg) | Number of tablets (250 mg) |
|
| Kg | ||
|
||
| 10 – 12 | 2 | 1 |
| 13 – 18 | 3 | 2 |
| 19 – 20 | 4 | 2 |
| ≥21 | 4 – 5* | 3† |
| Body weight | Twice daily (BID) 45 – 55 mg/kg | Three times daily (TID) 25 – 35 mg/kg |
||
|---|---|---|---|---|
|
||||
| Kg | Scoops of powder (50 mg/1 g) | Teaspoons* of powder | Scoops of powder (50 mg/1 g) | Teaspoons* of powder |
| 9.0 to <10.5 | 10 | 2½ | 6 | 1½ |
| 10.5 to <12 | 11 | 2¾ | 7 | 1¾ |
| 12 to <14 | 13 | 3¼ | 8 | 2 |
| 14 to <16 | 15 | 3¾ | 9 | 2¼ |
| 16 to <18 | Not recommended† | Not recommended† | 10 | 2½ |
| 18 to <23 | Not recommended† | Not recommended† | 12 | 3 |
| ≥23 | Not recommended† | Not recommended† | 15 | 3¾ |
2.4 Hepatic Impairment
VIRACEPT can be used in patients with mild hepatic impairment without any dose adjustment. VIRACEPT should not be used in patients with either moderate or severe hepatic impairment [see Warnings and Precautions (5.2), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
VIRACEPT 250 mg Tablet: Light-blue, capsule-shaped tablets with a clear film coating engraved with "VIRACEPT" on one side and "250 mg" on the other.
VIRACEPT 625 mg Tablet: White oval tablet with a clear film coating engraved with "V" on one side and "625" on the other.
VIRACEPT Oral Powder: Off-white powder containing 50 mg (as nelfinavir-free base) in each level scoopful (1 gram).
4. Contraindications
Coadministration of VIRACEPT is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events. These drugs and other contraindicated drugs (which may lead to reduced efficacy of nelfinavir) are listed in Table 3 [also see Drug Interactions (7), Table 6].
| Drug Class | Drugs Within Class That Are Contraindicated With VIRACEPT | Clinical Comment |
|---|---|---|
|
||
| Alpha 1-adrenoreceptor antagonist | Alfuzosin | Potentially increased alfuzosin concentrations can result in hypotension. |
| Antiarrhythmics | Amiodarone, quinidine | Potential for serious and/or life-threatening cardiac arrhythmia. |
| Antimycobacterial Agents | Rifampin | Plasma concentrations of nelfinavir can be reduced by concomitant use of rifampin. This may lead to loss of therapeutic effect and possible development of resistance to VIRACEPT or other coadministered antiretroviral agents. |
| Antipsychotics | Lurasidone Pimozide | Potential for serious and/or life-threatening reactions. Potential for serious and/or life threatening reactions such as cardiac arrhythmias. |
| Ergot Derivatives | Dihydroergotamine, ergotamine, methylergonovine | Potential for serious and/or life threatening reactions such as ergot toxicity characterized by peripheral vasospasm and ischemia of the extremities and other tissues. |
| GI Motility Agent | Cisapride | Potential for serious and/or life threatening reactions such as cardiac arrhythmias. |
| Herbal products | St. John's wort (Hypericum perforatum) | Plasma concentrations of nelfinavir can be reduced by concomitant use of the herbal preparation St. John's wort. This may lead to loss of therapeutic effect and possible development of resistance to VIRACEPT or other coadministered antiretroviral agents. |
| HMG-CoA Reductase Inhibitors | Lovastatin, Simvastatin | Potential for serious reactions such as myopathy including rhabdomyolysis. |
| PDE5 Inhibitors | Sildenafil (Revatio®) [for treatment of pulmonary arterial hypertension]* | A safe and effective dose has not been established when used with nelfinavir. There is increased potential for sildenafil-associated adverse events (which include visual disturbances, hypotension, prolonged erection, and syncope). |
| Sedative/Hypnotics | Triazolam, oral midazolam | Potential for serious and/or life threatening reactions such as prolonged or increased sedation or respiratory depression. |
5. Warnings and Precautions
ALERT: Find out about medicines that should not be taken with VIRACEPT. This statement is included on the product's bottle label.
5.1 Risk of Serious Adverse Reactions Due to Drug Interactions
Initiation of VIRACEPT, a CYP3A inhibitor, in patients receiving medications metabolized by CYP3A or initiation of medications metabolized by CYP3A in patients already receiving VIRACEPT, may increase plasma concentrations of medications metabolized by CYP3A. Initiation of medications that inhibit or induce CYP3A may increase or decrease concentrations of VIRACEPT, respectively. These interactions may lead to:
- Clinically significant adverse reactions, potentially leading to severe, life threatening, or fatal events from greater exposures of concomitant medications.
- Clinically significant adverse reactions from greater exposures of VIRACEPT.
- Loss of therapeutic effect of VIRACEPT and possible development of resistance.
See Table 6 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations [see Drug Interactions (7)]. Consider the potential for drug interactions prior to and during VIRACEPT therapy; review concomitant medications during VIRACEPT therapy; and monitor for the adverse reactions associated with the concomitant medications [see Contraindications (4) and Drug Interactions (7)].
5.2 Hepatic Impairment
VIRACEPT should not be used in patients with either moderate or severe hepatic impairment (Child-Pugh B or C, score greater than or equal to 7) [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
5.3 Phenylketonurics
Viracept Oral Powder contains phenylalanine, a component of aspartame. Each gram of VIRACEPT powder contains 11.2 mg phenylalanine. Phenylalanine can be harmful to patients with phenylketonuria.
5.4 Diabetes Mellitus/Hyperglycemia
New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus and hyperglycemia have been reported during post-marketing surveillance in HIV-infected patients receiving protease inhibitor therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases diabetic ketoacidosis has occurred. In those patients who discontinued protease inhibitor therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and a causal relationship between protease inhibitor therapy and these events has not been established.
5.5 Hemophilia
There have been reports of increased bleeding, including spontaneous skin hematomas and hemarthrosis, in patients with hemophilia type A and B treated with protease inhibitors. In some patients, additional factor VIII was given. In more than half of the reported cases, treatment with protease inhibitors was continued or reintroduced. A causal relationship has not been established.
5.6 Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement ("buffalo hump"), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
5.7 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including VIRACEPT. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections [such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jiroveci pneumonia (PCP), or tuberculosis], which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
6. Adverse Reactions/Side Effects
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Clinical Trials Experience: Adults and Adolescents (13 years and older)
The safety of VIRACEPT was studied in over 5000 patients who received drug either alone or in combination with nucleoside analogues. The majority of adverse events were of mild intensity. The most frequently reported adverse event among patients receiving VIRACEPT was diarrhea, which was generally of mild to moderate intensity.
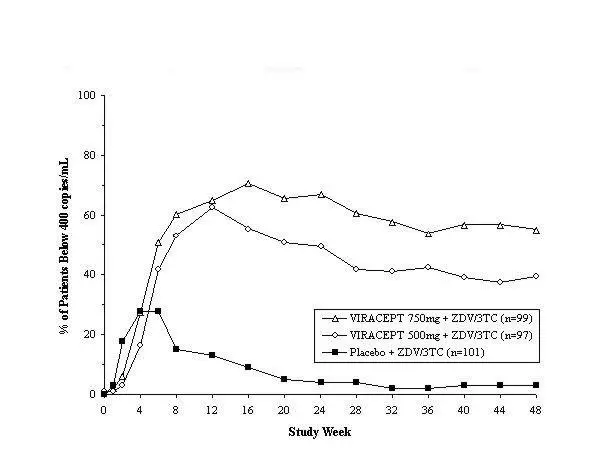
Drug-related clinical adverse experiences of moderate or severe intensity in ≥2% of patients treated with VIRACEPT coadministered with d4T and 3TC (Study 542) for up to 48 weeks, or with ZDV plus 3TC (Study 511) for up to 24 weeks are presented in Table 4.
| Study 511 | Study 542 | ||||
|---|---|---|---|---|---|
| 24 weeks | 48 weeks | ||||
| Adverse Events | Placebo + ZDV/3TC (n=101) | 500 mg TID VIRACEPT + ZDV/3TC (n=97) | 750 mg TID VIRACEPT + ZDV/3TC (n=100) | 1250 mg BID VIRACEPT + d4T/3TC (n=344) | 750 mg TID VIRACEPT + d4T/3TC (n=210) |
|
|||||
| Digestive System | |||||
| Diarrhea | 3% | 14% | 20% | 20% | 15% |
| Nausea | 4% | 3% | 7% | 3% | 3% |
| Flatulence | 0 | 5% | 2% | 1% | 1% |
| Skin/Appendages | |||||
| Rash | 1% | 1% | 3% | 2% | 1% |
Adverse events occurring in less than 2% of patients receiving VIRACEPT in all phase 2 and 3 clinical trials and considered at least possibly related or of unknown relationship to treatment and of at least moderate severity are listed below.
Body as a Whole: abdominal pain, accidental injury, allergic reaction, asthenia, back pain, fever, headache, malaise, pain, and redistribution/accumulation of body fat [see Warnings and Precautions (5.7)].
Digestive System: anorexia, dyspepsia, epigastric pain, gastrointestinal bleeding, hepatitis, mouth ulceration, pancreatitis, and vomiting.
Hemic/Lymphatic System: anemia, leukopenia, and thrombocytopenia.
Metabolic/Nutritional System: increases in alkaline phosphatase, amylase, creatine phosphokinase, lactic dehydrogenase, SGOT, SGPT, and gamma-glutamyl transpeptidase; hyperlipemia, hyperuricemia, hyperglycemia, hypoglycemia, dehydration, and liver function tests abnormal.
Musculoskeletal System: arthralgia, arthritis, cramps, myalgia, myasthenia, and myopathy.
Nervous System: anxiety, depression, dizziness, emotional lability, hyperkinesia, insomnia, migraine, paresthesia, seizures, sleep disorder, somnolence, and suicide ideation.
Respiratory System: dyspnea, pharyngitis, rhinitis, and sinusitis.
Skin/Appendages: dermatitis, folliculitis, fungal dermatitis, maculopapular rash, pruritus, sweating, and urticaria.
Special Senses: acute iritis and eye disorder.
Urogenital System: kidney calculus, sexual dysfunction, and urine abnormality.
Laboratory Abnormalities
The percentage of patients with marked laboratory abnormalities in Studies 542 and 511 are presented in Table 5. Marked laboratory abnormalities are defined as a Grade 3 or 4 abnormality in a patient with a normal baseline value, or a Grade 4 abnormality in a patient with a Grade 1 abnormality at baseline.
| Study 511 | Study 542 | ||||
|---|---|---|---|---|---|
| Placebo + ZDV/3TC (n=101) | 500 mg TID VIRACEPT + ZDV/3TC (n=97) | 750 mg TID VIRACEPT + ZDV/3TC (n=100) | 1250 mg BID VIRACEPT + d4T/3TC (n=344) | 750 mg TID VIRACEPT + d4T/3TC (n=210) |
|
|
|||||
| Hematology | |||||
| Hemoglobin | 6% | 3% | 2% | 0 | 0 |
| Neutrophils | 4% | 3% | 5% | 2% | 1% |
| Lymphocytes | 1% | 6% | 1% | 1% | 0 |
| Chemistry | |||||
| ALT (SGPT) | 6% | 1% | 1% | 2% | 1% |
| AST (SGOT) | 4% | 1% | 0 | 2% | 1% |
| Creatine Kinase | 7% | 2% | 2% | NA | NA |
6.2 Clinical Trials Experience: Pediatrics (2 to less than 13 years of age)
VIRACEPT has been studied in approximately 400 pediatric patients in clinical trials from birth to 13 years of age. The adverse event profile seen during pediatric clinical trials was similar to that for adults.
The most commonly reported drug-related, treatment-emergent adverse events reported in the pediatric studies included: diarrhea, leukopenia/neutropenia, rash, anorexia, and abdominal pain. Diarrhea, regardless of assigned relationship to study drug, was reported in 39% to 47% of pediatric patients receiving VIRACEPT in 2 of the larger treatment trials. Leukopenia/neutropenia was the laboratory abnormality most commonly reported as a significant event across the pediatric studies.
6.3 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of VIRACEPT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: hypersensitivity reactions (including bronchospasm, moderate to severe rash, fever, and edema).
Cardiovascular System: QTc prolongation, torsades de pointes.
Digestive System: jaundice.
Metabolic/Nutritional System: bilirubinemia, metabolic acidosis.
7. Drug Interactions
7.1 Potential for VIRACEPT to Affect Other Drugs
Nelfinavir is an inhibitor of CYP3A. Coadministration of VIRACEPT and drugs primarily metabolized by CYP3A (e.g., dihydropyridine calcium channel blockers, HMG-CoA reductase inhibitors, immunosuppressants and PDE5 inhibitors) may result in increased plasma concentrations of such drugs that could increase or prolong both its therapeutic and adverse effects. (see Tables 3 and 6).
7.2 Potential for Other Drugs to Affect VIRACEPT
Nelfinavir is metabolized by CYP3A and CYP2C19. Coadministration of VIRACEPT and drugs that induce CYP3A or CYP2C19, such as rifampin, may decrease nelfinavir plasma concentrations and reduce its therapeutic effect. Coadministration of VIRACEPT and drugs that inhibit CYP3A or CYP2C19 may increase nelfinavir plasma concentrations.
7.3 Established and Other Potentially Significant Drug Interactions
Table 6 provides the effect on concentrations of VIRACEPT or concomitant drug as a result of coadministration with VIRACEPT. These recommendations are based on either drug interaction studies or predicted interactions due to the expected magnitude of interaction and potential for serious adverse events or loss of efficacy.
| Concomitant Drug Class: Drug Name | Effect on Concentration | Clinical Comment |
|---|---|---|
| HIV Antiviral Agents: Reverse Transcriptase Inhibitors | ||
| Delavirdine | ↑ nelfinavir (Cmin) ↓ delavirdine | Concentrations of nelfinavir were increased while concentrations of delavirdine were decreased when the two agents were coadministered. Appropriate doses of the combination, with respect to safety and efficacy, have not been established. |
| Nevirapine | ↓ nelfinavir (Cmin) | Concentrations of nelfinavir were decreased when coadministered with nevirapine. An appropriate dose of nelfinavir with respect to safety and efficacy has not been established. |
| Didanosine | ↔ nelfinavir | There was no change in nelfinavir concentration when coadministered with didanosine. However, it is recommended that didanosine be administered on an empty stomach; therefore, didanosine should be given one hour before or two hours after VIRACEPT (given with food). |
| HIV Antiviral Agents: Protease Inhibitors | ||
| Indinavir | ↑ nelfinavir ↑ indinavir | Concentrations of both indinavir and nelfinavir were increased when the two agents were coadministered. Appropriate doses for these combinations, with respect to safety and efficacy, have not been established. |
| Ritonavir | ↑ nelfinavir ↔ ritonavir | Concentrations of nelfinavir were increased when coadministered with ritonavir. An appropriate dose of nelfinavir for this combination, with respect to safety and efficacy, has not been established. |
| Saquinavir | ↑ nelfinavir ↑ saquinavir | Concentrations of both saquinavir and nelfinavir were increased when the two agents were coadministered. Appropriate doses for these combinations, with respect to safety and efficacy, have not been established. |
| ANTICOAGULANT | ||
| Warfarin | Warfarin | Coadministration of warfarin and VIRACEPT may affect concentrations of warfarin. It is recommended that the INR (international normalized ratio) be monitored carefully during treatment with VIRACEPT, especially when commencing therapy. |
| ANTICONVULSANTS | ||
| Carbamazepine Phenobarbital Phenytoin |
↓ nelfinavir ↓ phenytoin | Concentrations of nelfinavir may be decreased. VIRACEPT may not be effective due to decreased nelfinavir plasma concentrations in patients taking these agents concomitantly. Phenytoin plasma/serum concentrations should be monitored; phenytoin dose may require adjustment to compensate for altered phenytoin concentration. |
| ANTIDEPRESSANT | ||
| Trazodone | ↑ trazodone | Concomitant use of trazodone and VIRACEPT may increase plasma concentrations of trazodone. Adverse events of nausea, dizziness, hypotension and syncope have been observed following coadministration of trazodone and ritonavir. If trazodone is used with a CYP3A4 inhibitor such as VIRACEPT, the combination should be used with caution and a lower dose of trazodone should be considered. |
| ANTIGOUT | ||
| Colchicine | ↑ colchicines | Patients with renal or hepatic impairment should not be given colchicine with VIRACEPT due to the risk of colchicine toxicity. Treatment of gout flares – co- administration of colchicine in patients on VIRACEPT: 0.6 mg (1 tablet) × 1 dose, followed by 0.3 mg (half tablet) 1 hour later. Dose to be repeated no earlier than 3 days. Prophylaxis of gout-flares – coadministration of colchicine in patients on VIRACEPT: If the original colchicine regimen was 0.6 mg twice a day, the regimen should be adjusted to 0.3 mg once a day. If the original colchicine regimen was 0.6 mg once a day, the regimen should be adjusted to 0.3 mg once every other day. Treatment of familial Mediterranean fever (FMF)– coadministration of colchicine in patients on VIRACEPT: Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day). |
| ANTIMYCOBACTERIAL | ||
| Rifabutin | ↑ rifabutin ↓ nelfinavir (750 mg TID) ↔ nelfinavir (1250 mg BID) | It is recommended that the dose of rifabutin be reduced to one-half the usual dose when administered with VIRACEPT; 1250 mg BID is the preferred dose of VIRACEPT when coadministered with rifabutin. |
| ENDOTHELIN RECEPTOR ANTAGONIST | ||
| Bosentan | ↑ bosentan | Concentrations of bosentan may be increased when coadministered with VIRACEPT. Coadministration of bosentan in patients on VIRACEPT or coadministration of VIRACEPT in patients on bosentan: Start at or adjust bosentan to 62.5 mg once daily or every other day based upon individual tolerability. |
| HMG-CoA REDUCTASE INHIBITORS | ||
| Atorvastatin Rosuvastatin | ↑ atorvastatin ↑ rosuvastatin | Titrate atorvastatin dose carefully and use the lowest necessary dose; do not exceed atorvastatin 40 mg/day. |
| IMMUNOSUPPRESSANTS | ||
| Cyclosporine Tacrolimus Sirolimus | ↑ immuno-suppressants ↑ nelfinavir | Concentrations of these immunosuppressants and nelfinavir may be increased by coadministration of these agents with nelfinavir. |
| INHALED BETA AGONIST | ||
| Salmeterol | ↑ salmeterol | Concurrent administration of salmeterol with VIRACEPT is not recommended. The combination may result in increased risk of cardiovascular adverse events associated with salmeterol, including QT prolongation, palpitations and sinus tachycardia. |
| INHALED/NASAL STEROID | ||
| Fluticasone | ↑ fluticasone | Concomitant use of fluticasone propionate and VIRACEPT may increase plasma concentrations of fluticasone propionate. Use with caution. Consider alternatives to fluticasone propionate, particularly for long-term use. |
| MACROLIDE ANTIBIOTIC | ||
| Azithromycin | ↑ azithromycin | Dose adjustment of azithromycin is not recommended, but close monitoring for known side effects such as liver enzyme abnormalities and hearing impairment is warranted. |
| NARCOTIC ANALGESIC | ||
| Methadone | ↓ methadone | Concentrations of methadone were decreased when coadministered with VIRACEPT. Dosage of methadone may need to be increased when coadministered with VIRACEPT. |
| HORMONAL CONTRACEPTIVES | ||
| Ethinyl estradiol Norethindrone | ↓ ethinyl estradiol ↓ norethindrone | Concentrations of ethinyl estradiol and norethindrone were decreased when coadministered with VIRACEPT. Alternative or additional contraceptive measures should be used when oral contraceptives containing ethinyl estradiol or norethindrone and VIRACEPT are coadministered. |
| PDE5 INHIBITORS | ||
| Sildenafil Vardenafil Tadalafil | ↑ PDE5 Inhibitors | Concomitant use of PDE5 inhibitors and VIRACEPT should be undertaken with caution. May result in an increase in PDE5 inhibitor-associated adverse events, including hypotension, syncope, visual disturbances, and priapism. Use of PDE5 inhibitors for pulmonary arterial hypertension (PAH): • Use of sildenafil (REVATIO) is contraindicated when used for the treatment of pulmonary arterial hypertension (PAH) [see Contraindications (4)]. • The following dose adjustments are recommended for use of tadalafil (ADCIRCA™) with VIRACEPT: Coadministration of ADCIRCA in patients on VIRACEPT or coadministration of VIRACEPT in patients on ADCIRCA: Start at or adjust ADCIRCA to 20 mg once daily. Increase to 40 mg once daily based upon individual tolerability. Use of PDE5 inhibitors for erectile dysfunction: Sildenafil at a single dose not exceeding 25 mg in 48 hours, vardenafil at a single dose not exceeding 2.5 mg in 24 hours, or tadalafil at a single dose not exceeding 10 mg dose in 72 hours, is recommended. Use with increased monitoring for adverse events. |
| PROTON PUMP INHIBITORS | ||
| Omeprazole | ↓ nelfinavir | Omeprazole decreases the plasma concentrations of nelfinavir. Concomitant use of proton pump inhibitors and VIRACEPT may lead to a loss of virologic response and development of resistance. |
| ANTIPSYCHOTICS | ||
| Quetiapine | ↑ quetiapine | Initiation of VIRACEPT in patients taking quetiapine:
Consider alternative antiretroviral therapy to avoid increases in quetiapine drug exposures. If coadministration is necessary, reduce the quetiapine dose to 1/6 of the current dose and monitor for quetiapine-associated adverse reactions. Refer to the quetiapine prescribing information for recommendations on adverse reaction monitoring. Initiation of quetiapine in patients taking VIRACEPT: Refer to the quetiapine prescribing information for initial dosing and titration of quetiapine. |
8. Use In Specific Populations
8.4 Pediatric Use
The safety, tolerability, pharmacokinetic profile and efficacy of VIRACEPT were evaluated in HIV infected pediatric patients from 2 to 13 years of age in multicenter clinical trials, Study 556 and PACTG 337 [see Clinical Pharmacology (12.3) and Clinical Studies (14.3)]. In patients less than 2 years of age, VIRACEPT was found to be safe at the doses studied, but a reliably effective dose could not be established [see Dosage and Administration (2.2), Adverse Reactions (6.2), and Clinical Pharmacology (12.3)]. The pharmacokinetic profile, safety and antiviral activity of VIRACEPT in adolescent patients 13 years and older is supported by data from the adult clinical trials where some trials allowed enrolment of subjects 13 years and older. Thus, the data for adolescents and adults were analyzed collectively. [see Adverse Reactions (6.1)].
8.5 Geriatric Use
Clinical studies of VIRACEPT did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
8.6 Hepatic Impairment
VIRACEPT should not be used in patients with either moderate or severe hepatic impairment (Child-Pugh B or C, score greater than or equal to 7) [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)]. No dose adjustment of VIRACEPT is necessary for patients with mild hepatic impairment (Child-Pugh A, score 5–6).
10. Overdosage
Human experience of acute overdose with VIRACEPT is limited. There is no specific antidote for overdose with VIRACEPT. If indicated, elimination of unabsorbed drug should be achieved by emesis or gastric lavage. Administration of activated charcoal may also be used to aid removal of unabsorbed drug. Since nelfinavir is highly protein bound, dialysis is unlikely to significantly remove drug from blood.
11. Viracept Description
VIRACEPT® (nelfinavir mesylate) is an inhibitor of the human immunodeficiency virus type 1 (HIV-1) protease. VIRACEPT Tablets are available for oral administration as a light-blue, capsule-shaped tablet with a clear film coating in 250 mg strength (as nelfinavir-free base) and as a white oval tablet with a clear film coating in 625 mg strength (as nelfinavir-free base). Each tablet contains the following common inactive ingredients: calcium silicate, crospovidone, magnesium stearate, hypromellose, and triacetin. In addition, the 250 mg tablet contains FD&C blue #2 powder and the 625 mg tablet contains colloidal silicon dioxide. VIRACEPT Oral Powder is available for oral administration in a 50 mg/g strength (as nelfinavir-free base) in bottles. The oral powder also contains the following inactive ingredients: microcrystalline cellulose, maltodextrin, dibasic potassium phosphate, crospovidone, hypromellose, aspartame, sucrose palmitate, and natural and artificial flavor. The chemical name for nelfinavir mesylate is [3S-[2(2S*, 3S*), 3α,4aβ,8aβ]]-N-(1,1-dimethylethyl)decahydro-2-[2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylthio)butyl]-3-isoquinoline carboxamide mono-methanesulfonate (salt) and the molecular weight is 663.90 (567.79 as the free base). Nelfinavir mesylate has the following structural formula:
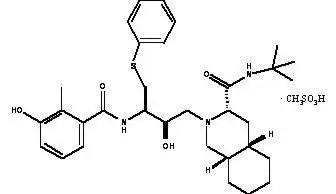
Nelfinavir mesylate is a white to off-white amorphous powder, slightly soluble in water at pH ≤4 and freely soluble in methanol, ethanol, 2-propanol and propylene glycol.
12. Viracept - Clinical Pharmacology
12.1 Mechanism of Action
Nelfinavir is an inhibitor of the HIV-1 protease [see Microbiology (12.4)].
12.3 Pharmacokinetics
The pharmacokinetic properties of nelfinavir were evaluated in healthy volunteers and HIV-infected patients; no substantial differences were observed between the two groups.
Absorption
Pharmacokinetic parameters of nelfinavir (area under the plasma concentration-time curve during a 24-hour period at steady-state [AUC24], peak plasma concentrations [Cmax], morning and evening trough concentrations [Ctrough]) from a pharmacokinetic study in HIV-positive patients after multiple dosing with 1250 mg (five 250 mg tablets) twice daily (BID) for 28 days (10 patients) and 750 mg (three 250 mg tablets) three times daily (TID) for 28 days (11 patients) are summarized in Table 7.
| Regimen | AUC24
mg∙h/L | Cmax
mg/L | Ctrough
Morning mg/L | Ctrough
Afternoon or Evening mg/L |
|---|---|---|---|---|
| Data are mean ± SD | ||||
| 1250 mg BID | 52.8±15.7 | 4.0±0.8 | 2.2±1.3 | 0.7±0.4 |
| 750 mg TID | 43.6±17.8 | 3.0±1.6 | 1.4±0.6 | 1.0±0.5 |
The difference between morning and afternoon or evening trough concentrations for the TID and BID regimens was also observed in healthy volunteers who were dosed at precisely 8- or 12-hour intervals.
In healthy volunteers receiving a single 1250 mg dose, the 625 mg tablet was not bioequivalent to the 250 mg tablet formulation. Under fasted conditions (n=27), the AUC and Cmax were 34% and 24% higher, respectively, for the 625 mg tablets. In a relative bioavailability assessment under fed conditions (n=28), the AUC was 24% higher for the 625 mg tablet; the Cmax was comparable for both formulations. In HIV-1 infected subjects (N=21) receiving multiple doses of 1250 mg BID under fed conditions, the 625 mg formulation was bioequivalent to the 250 mg formulation based on similarity in steady state exposure (Cmax and AUC).
Table 8 shows the summary of the steady state pharmacokinetic parameters (mean ± SD) of nelfinavir after multiple dose administration of 1250 mg BID (2 × 625 mg tablets) to HIV-infected patients (N=21) for 14 days.
| Regimen | AUC12
mg∙h/L | Cmax
mg/L | Cmin
mg/L |
|---|---|---|---|
| AUC12: Steady state AUC | |||
| Cmax: Maximum plasma concentration at steady state | |||
| Cmin: Minimum plasma concentration at steady state | |||
| 1250 mg BID | 35.3 (16.4) | 4.7 (1.9) | 1.5 (1.0) |
In healthy volunteers receiving a single 750 mg dose under fed conditions, nelfinavir concentrations were similar following administration of the 250 mg tablet and oral powder.
Effect of Food on Oral Absorption
Food increases nelfinavir exposure and decreases nelfinavir pharmacokinetic variability relative to the fasted state. In one study, healthy volunteers received a single dose of 1250 mg of VIRACEPT 250 mg tablets (5 tablets) under fasted or fed conditions (three different meals). In a second study, healthy volunteers received single doses of 1250 mg VIRACEPT (5 × 250 mg tablets) under fasted or fed conditions (two different fat content meals). The results from the two studies are summarized in Table 9 and Table 10, respectively.
| Number of Kcal | % Fat | Number of subjects | AUC fold increase | Cmax fold increase | Increase in Tmax (hr) |
|---|---|---|---|---|---|
| 125 | 20 | n=21 | 2.2 | 2.0 | 1.00 |
| 500 | 20 | n=22 | 3.1 | 2.3 | 2.00 |
| 1000 | 50 | n=23 | 5.2 | 3.3 | 2.00 |
| Number of Kcal | % Fat | Number of subjects | AUC fold increase | Cmax fold increase | Increase in Tmax (hr) |
|---|---|---|---|---|---|
| 500 | 20 | n=22 | 3.1 | 2.5 | 1.8 |
| 500 | 50 | n=22 | 5.1 | 3.8 | 2.1 |
Nelfinavir exposure can be increased by increasing the calorie or fat content in meals taken with VIRACEPT.
A food effect study has not been conducted with the 625 mg tablet. However, based on a cross-study comparison (n=26 fed vs. n=26 fasted) following single dose administration of nelfinavir 1250 mg, the magnitude of the food effect for the 625 mg nelfinavir tablet appears comparable to that of the 250 mg tablets. VIRACEPT should be taken with a meal.
Specific Populations
Drug Interactions
CYP3A and CYP2C19 appear to be the predominant enzymes that metabolize nelfinavir in humans. The potential ability of nelfinavir to inhibit the major human cytochrome P450 enzymes (CYP3A, CYP2C19, CYP2D6, CYP2C9, CYP1A2 and CYP2E1) has been investigated in vitro. Only CYP3A was inhibited at concentrations in the therapeutic range. Specific drug interaction studies were performed with nelfinavir and a number of drugs. Table 12 summarizes the effects of nelfinavir on the geometric mean AUC, Cmax and Cmin of coadministered drugs. Table 13 shows the effects of coadministered drugs on the geometric mean AUC, Cmax and Cmin of nelfinavir.
| % Change of Coadministered Drug Pharmacokinetic Parameters* (90% CI) | |||||
|---|---|---|---|---|---|
| Coadministered Drug | Nelfinavir Dose | N | AUC | Cmax | Cmin |
| NA: Not relevant for single-dose treatment; ND: Cannot be determined | |||||
|
|||||
| HIV-Protease Inhibitors | |||||
| Indinavir 800 mg Single Dose | 750 mg q8h × 7 days | 6 | ↑51% (↑29–↑77%) | ↓10% (↓28–↑13%) | NA |
| Ritonavir 500 mg Single Dose | 750 mg q8h × 5 doses | 10 | ↔ | ↔ | NA |
| Saquinavir 1200 mg Single Dose† | 750 mg TID × 4 days | 14 | ↑392% (↑291–↑521%) | ↑179% (↑117–↑259%) | NA |
| Amprenavir 800 mg TID × 14 days | 750 mg TID × 14 days | 6 | ↔ | ↓14% (↓38–↑20%) | ↑189% (↑52–↑448%) |
| Nucleoside Reverse Transcriptase Inhibitors | |||||
| Lamivudine 150 mg Single Dose | 750 mg q8h × 7–10 days | 11 | ↑10% (↑2–↑18%) | ↑31% (↑9–↑56%) | NA |
| Zidovudine 200 mg Single Dose | 750 mg q8h × 7–10 days | 11 | ↓35% (↓29–↓40%) | ↓31% (↓13–↓46%) | NA |
| Non-nucleoside Reverse Transcriptase Inhibitors | |||||
| Efavirenz 600 mg qd × 7 days | 750 mg q8h × 7 days | 10 | ↓12% (↓31–↑12%) | ↓12% (↓29–↑8%) | ↓22% (↓54–↑32%) |
| Delavirdine 400 mg q8h × 14 days | 750 mg q8h × 7 days | 7 | ↓31% (↓57–↑10%) | ↓27% (↓49–↑4%) | ↓33% (↓70–↑49%) |
| Anti-infective Agents | |||||
| Rifabutin 150 mg qd × 8 days‡ | 750 mg q8h × 7–8 days§ | 12 | ↑83% (↑72–↑96%) | ↑19% (↑11–↑28%) | ↑177% (↑144–↑215%) |
| Rifabutin 300 mg qd × 8 days | 750 mg q8h × 7–8 days | 10 | ↑207% (↑161–↑263%) | ↑146% (↑118–↑178%) | ↑305% (↑245–↑375%) |
| Azithromycin 1200 mg Single Dose | 750 mg TID × 11 days | 12 | ↑112% (↑80–↑150%) | ↑136% (↑77–↑215%) | NA |
| HMG-CoA Reductase Inhibitors | |||||
| Atorvastatin 10 mg qd × 28 days | 1250 mg BID × 14 days | 15 | ↑74% (↑41–↑116%) | ↑122% (↑68–↑193%) | ↑39% (↓21–↑145%) |
| Simvastatin 20 mg qd × 28 days | 1250 mg BID × 14 days | 16 | ↑505% (↑393–↑643%) | ↑517% (↑367–↑715%) | ND |
| Other Agents | |||||
| Ethinyl estradiol 35 µg qd × 15 days | 750 mg q8h × 7 days | 12 | ↓47% (↓42–↓52%) | ↓28% (↓16–↓37%) | ↓62% (↓57–↓67%) |
| Norethindrone 0.4 mg qd × 15 days | 750 mg q8h × 7 days | 12 | ↓18% (↓13–↓23%) | ↔ | ↓46% (↓38–↓53%) |
| Methadone 80 mg ± 21 mg qd¶ >1 month | 1250 mg BID × 8 days | 13 | ↓47% (↓42–↓51%) | ↓46% (↓42–↓49%) | ↓53% (↓49–↓57%) |
| Phenytoin 300 mg qd × 14 days# | 1250 mg BID × 7 days | 12 | ↓29% (↓17–↓39%) | ↓21% (↓12–↓29%) | ↓39% (↓27–↓49%) |
| % Change of Nelfinavir Pharmacokinetic Parameters* (90% CI) | |||||
|---|---|---|---|---|---|
| Coadministered Drug | Nelfinavir Dose | N | AUC | Cmax | Cmin |
| NA: Not relevant for single-dose treatment | |||||
|
|||||
| HIV-Protease Inhibitors | |||||
| Indinavir 800 mg q8h × 7 days | 750 mg Single Dose | 6 | ↑83% (↑42–↑137%) | ↑31% (↑16–↑48%) | NA |
| Ritonavir 500 mg q12h × 3 doses | 750 mg Single Dose | 10 | ↑152% (↑96–↑224%) | ↑44% (↑28–↑63%) | NA |
| Saquinavir 1200 mg TID × 4 days† | 750 mg Single Dose | 14 | ↑18% (↑7–↑30%) | ↔ | NA |
| Nucleoside Reverse Transcriptase Inhibitors | |||||
| Didanosine 200 mg Single Dose | 750 mg Single Dose | 9 | ↔ | ↔ | NA |
| Zidovudine 200 mg + Lamivudine 150 mg Single Dose | 750 mg q8h × 7–10 days | 11 | ↔ | ↔ | ↔ |
| Non-nucleoside Reverse Transcriptase Inhibitors | |||||
| Efavirenz 600 mg qd × 7 days | 750 mg q8h × 7 days | 7 | ↑20% (↑8–↑34%) | ↑21% (↑10–↑33%) | ↔ |
| Nevirapine 200 mg qd × 14 days followed by 200 mg BID × 14 days | 750 mg TID × 36 days | 23 | ↔ | ↔ | ↓32% (↓50–↑5%) |
| Delavirdine 400 mg q8h × 7 days | 750 mg q8h × 14 days | 12 | ↑107% (↑83–↑135%) | ↑88% (↑66–↑113%) | ↑136% (↑103–↑175%) |
| Anti-infective Agents | |||||
| Ketoconazole 400 mg qd × 7 days | 500 mg q8h × 5–6 days | 12 | ↑35% (↑24–↑46%) | ↑25% (↑11–↑40%) | ↑14% (↓23–↑69%) |
| Rifabutin 150 mg qd × 8 days | 750 mg q8h × 7–8 days | 11 | ↓23% (↓14–↓31%) | ↓18% (↓8–↓27%) | ↓25% (↓8–↓39%) |
| 1250 mg q12h × 7–8 days | 11 | ↔ | ↔ | ↓15% (↓43–↑27%) |
|
| Rifabutin 300 mg qd × 8 days | 750 mg q8h × 7–8 days | 10 | ↓32% (↓15–↓46%) | ↓24% (↓10–↓36%) | ↓53% (↓15–↓73%) |
| Rifampin 600 mg qd × 7 days | 750 mg q8h × 5–6 days | 12 | ↓83% (↓79–↓86%) | ↓76% (↓69–↓82%) | ↓92% (↓86–↓95%) |
| Azithromycin 1200 mg Single Dose | 750 mg tid × 9 days | 12 | ↓15% (↓7–↓22%) | ↓10% (↓19–↑1%) | ↓29% (↓19–↓38%) |
| Other Agents | |||||
| Phenytoin 300 mg qd × 7 days | 1250 mg BID × 14 days | 15 | ↔ | ↔ | ↓18% (↓45–↑23%) |
| Omeprazole 40 mg qd × 4 days administered 30 minutes before nelfinavir | 1250 mg BID × 4 days | 19 | ↓36% (↓20–↓49%) | ↓37% (↓23–↓49%) | ↓39% (↓15–↓57%) |
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies in mice and rats were conducted with nelfinavir at oral doses up to 1000 mg/kg/day. No evidence of a tumorigenic effect was noted in mice at systemic exposures (Cmax) up to 9-fold those measured in humans at the recommended therapeutic dose (750 mg TID or 1250 mg BID). In rats, thyroid follicular cell adenomas and carcinomas were increased in males at 300 mg/kg/day and higher and in females at 1000 mg/kg/day. Systemic exposures (Cmax) at 300 and 1000 mg/kg/day were 1- to 3-fold, respectively, those measured in humans at the recommended therapeutic dose. Repeated administration of nelfinavir to rats produced effects consistent with hepatic microsomal enzyme induction and increased thyroid hormone deposition; these effects predispose rats, but not humans, to thyroid follicular cell neoplasms. Nelfinavir showed no evidence of mutagenic or clastogenic activity in a battery of in vitro and in vivo genetic toxicology assays. These studies included bacterial mutation assays in S. typhimurium and E. coli, a mouse lymphoma tyrosine kinase assay, a chromosomal aberration assay in human lymphocytes, and an in vivo mouse bone marrow micronucleus assay.
Nelfinavir produced no effects on either male or female mating and fertility or embryo survival in rats at systemic exposures comparable to the human therapeutic exposure.
14. Clinical Studies
Description of Clinical Studies
The efficacy of VIRACEPT is based on analyses of multiple clinical studies in HIV-1 infected antiretroviral treatment-naïve and experienced adult patients. In the adult clinical studies described below, efficacy was evaluated by the percent of patients with plasma HIV RNA <400 copies/mL (Studies 511 and 542) , <500 copies/mL (Study ACTG 364), or <50 copies/mL (Study Avanti 3). In the analysis presented in each figure, patients who terminated the study early for any reason, switched therapy due to inadequate efficacy or who had a missing HIV-RNA measurement that was either preceded or followed by a measurement above the limit of assay quantification were considered to have HIV-RNA above 400 copies/mL, above 500 copies/mL, or above 50 copies/mL at subsequent time points, depending on the study's definition of virologic failure.
14.1 Studies in Antiretroviral Treatment Naïve Adult Patients
Study 542: VIRACEPT BID + stavudine + lamivudine compared to VIRACEPT TID + stavudine + lamivudine
Study 542 is a, randomized, open-label trial comparing the HIV RNA suppression achieved by VIRACEPT 1250 mg BID versus VIRACEPT 750 mg TID in patients also receiving stavudine (d4T; 30–40 mg BID) and lamivudine (3TC; 150 mg BID). Patients had a median age of 36 years (range 18 to 83), were 84% male, and were 91% Caucasian. Patients had received less than 6 months of therapy with nucleoside transcriptase inhibitors and were naïve to protease inhibitors. Mean baseline CD4 cell count was 296 cells/mm3 and mean baseline plasma HIV RNA was 5.0 log10 copies/mL (100,706 copies/mL).
Results showed that there was no significant difference in mean CD4 cell count among treatment groups; the mean increases from baseline for the BID and TID arms were 150 cells/mm3 at 24 weeks and approximately 200 cells/mm3 at 48 weeks.
The percent of patients with HIV RNA <400 copies/mL and the outcomes of patients through 48 weeks of treatment are summarized in Table 14.
| Outcome | VIRACEPT 1250 mg BID Regimen | VIRACEPT 750 mg TID Regimen |
|---|---|---|
|
||
| Number of patients evaluable* | 323 | 192 |
| HIV-1 RNA <400 copies/mL | 198 (61%) | 111 (58%) |
| HIV-1 RNA ≥400 copies/mL | 46 (14%) | 22 (11%) |
| Discontinued due to VIRACEPT toxicity† | 9 (3%) | 2 (1%) |
| Discontinued due to other antiretroviral agents' toxicity† | 3 (1%) | 3 (2%) |
| Others‡ | 67 (21%) | 54 (28%) |
14.3 Studies in Pediatric Patients
The pharmacokinetic profile, safety and antiviral activity of VIRACEPT in pediatric patients 2 years of age up to 13 years were evaluated in 2 randomized studies.
Study 556 was a randomized, double-blind, placebo-controlled trial with VIRACEPT or placebo coadministered with ZDV and ddI in 141 HIV-positive children who had received minimal antiretroviral therapy. The mean age of the children was 3.9 years. Ninety four (67%) children were between 2–12 years, and 47 (33%) were < 2 years of age. The mean baseline HIV RNA value was 5.0 log for all patients and the mean CD4 cell count was 886 cells/mm3 for all patients. The efficacy of VIRACEPT measured by HIV RNA <400 at 48 weeks in children ≥2 years of age was 26% compared to 2% of placebo patients (p=0.0008). In the children < 2 years of age, only 1 of 27 and 2 of 20 maintained an undetectable HIV RNA level at 48 weeks for placebo and VIRACEPT patients, respectively.
PACTG 377 was an open-label study that randomized 181 HIV treatment-experienced pediatric patients to receive: d4T+NVP+RTV, d4T+3TC+NFV, or d4T+3TC+NVP+NFV with NFV given on a TID schedule. The median age was 5.9 years and 46% were male. At baseline the median HIV RNA was 4.4 log and median CD4 cell count was 690 cells/mm3. Substudy PACTG 725 evaluated d4T+3TC+NFV with NFV given on a BID schedule. The proportion of patients with detectable viral load at baseline achieving HIV RNA <400 copies/mL at 48 weeks was: 41% for d4T+NVP+RTV, 42% for d4T+3TC+NFV, 30% for d4T+NVP+NFV, and 52% for d4T+3TC+NVP+NFV. No significant clinical differences were identified between patients receiving VIRACEPT in BID or TID schedules.
VIRACEPT has been evaluated in 2 studies of young infants. The PENTA 7 study was an open-label study to evaluate the toxicity, tolerability, pharmacokinetics, and activity of NFV+d4T+ddI in 20 HIV-infected infants less than 12 weeks of age. PACTG 353 evaluated the pharmacokinetics and safety of VIRACEPT in infants born to HIV-infected women receiving NFV as part of combination therapy during pregnancy.
The following issues should be considered when initiating VIRACEPT in pediatric patients:
- In pediatric patients ≥2 years of age receiving VIRACEPT as part of triple combination antiretroviral therapy in randomized studies, the proportion of patients achieving a HIV RNA level <400 copies/mL through 48 weeks ranged from 26% to 42%.
- Response rates in children <2 years of age appeared to be poorer than those in patients ≥2 years of age in some studies.
- Highly variable drug exposure remains a significant problem in the use of VIRACEPT in pediatric patients. Unpredictable drug exposure may be exacerbated in pediatric patients because of increased clearance compared to adults and difficulties with compliance and adequate food intake with dosing. Pharmacokinetic results from the pediatric studies are reported in Table 11 [see Clinical Pharmacology (12.3)].
The pharmacokinetic profile, safety and antiviral activity of VIRACEPT in adolescent patients 13 years and older is supported by data from the adult clinical trials where some trials allowed enrolment of subjects 13 years and older. Thus, the data for adolescents and adults were analyzed collectively.
16. How is Viracept supplied
VIRACEPT (nelfinavir mesylate) 250 mg: Light-blue, capsule-shaped tablets with a clear film coating engraved with "VIRACEPT" on one side and "250 mg" on the other.
- Bottles of 300 (250 mg) tablets – NDC 63010-010-30
VIRACEPT (nelfinavir mesylate) 625 mg: White oval tablet with a clear film coating engraved with "V" on one side and "625" on the other.
- Bottles of 120 (625 mg) tablets – NDC 63010-027-70
VIRACEPT (nelfinavir mesylate) Oral Powder is available as a 50 mg/g off-white powder containing 50 mg (as nelfinavir free base) in each level scoopful (1 gram).
- Multiple use bottles of 144 grams of powder with scoop …….NDC 63010-011-90
17. Patient Counseling Information
See FDA-approved patient labeling (Patient Information)
A statement to patients and healthcare providers is included on the product's bottle label: ALERT: Find out about medicines that should NOT be taken with VIRACEPT.
- Instruction for Use
For optimal absorption, patients should be advised to take VIRACEPT with food.
Patients should be informed that VIRACEPT Tablets are film-coated and that this film-coating is intended to make the tablets easier to swallow.
If a dose of VIRACEPT is missed, patients should take the dose as soon as possible and then return to their normal schedule. However, if a dose is skipped, the patient should not double the next dose.
Adult or pediatric patients unable to swallow the tablets may dissolve the tablets in a small amount of water:
-
- Place VIRACEPT tablet(s) in small amount of water
- Once dissolved, mix the cloudy liquid well, and consume it immediately.
- The glass should be rinsed with water and the rinse swallowed to ensure the entire dose is consumed
Pediatric patients unable to swallow tablets can also use the powder formulation:
-
- Mix VIRACEPT Oral Powder with a small amount of water, milk, formula, soy formula, soy milk, or dietary supplements
- Once mixed, the entire contents must be consumed in order to obtain the full dose.
- If the mixture is not consumed immediately, it must be stored under refrigeration, but storage must not exceed 6 hours.
- Acidic food or juice (e.g., orange juice, apple juice, or apple sauce) are not recommended for mixing VIRACEPT Oral Powder because the combination may result in a bitter taste.
- VIRACEPT Oral Powder should not be reconstituted with water in its original container.
| VIRACEPT
nelfinavir mesylate tablet, film coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| VIRACEPT
nelfinavir mesylate tablet, film coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - AGOURON (145772760) |
| Registrant - Pfizer Inc (113480771) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmacia & Upjohn Company LLC | 618054084 | ANALYSIS(63010-010, 63010-027) , API MANUFACTURE(63010-010, 63010-027) , PACK(63010-010, 63010-027) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Manufacturing Deutschland GmbH | 341970073 | ANALYSIS(63010-010, 63010-027) , MANUFACTURE(63010-010, 63010-027) , PACK(63010-010, 63010-027) | |