Drug Detail:Vivjoa (Oteseconazole)
Drug Class: Azole antifungals
Highlights of Prescribing Information
VIVJOA™ (oteseconazole) capsules, for oral use
Initial U.S. Approval: 2022
Indications and Usage for Vivjoa
VIVJOA™ is an azole antifungal indicated to reduce the incidence of recurrent vulvovaginal candidiasis (RVVC) in females with a history of RVVC who are NOT of reproductive potential. ( 1)
Vivjoa Dosage and Administration
- There are two recommended VIVJOA dosage regimens: a VIVJOA-only regimen and a Fluconazole/VIVJOA regimen. Use one of these two dosage regimens. (
2.1)
- Administer VIVJOA orally with food. ( 2.1)
- For the
VIVJOA-only Dosage Regimen: (
2.2)
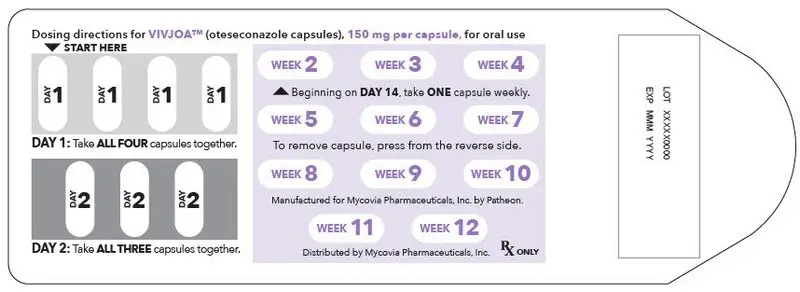
- On Day 1: Administer VIVJOA 600 mg (as a single dose), then
- On Day 2: Administer VIVJOA 450 mg (as a single dose), then
- Beginning on Day 14: Administer VIVJOA 150 mg once a week (every 7 days) for 11 weeks (Weeks 2 through 12).
- For the
Fluconazole/VIVJOA Dosage Regimen, prescribe fluconazole and: (
2.3)
- On Day 1, Day 4, and Day 7: Administer fluconazole 150 mg orally, then
- On Days 14 through 20: Administer VIVJOA 150 mg once daily for 7 days, then
- Beginning on Day 28: Administer VIVJOA 150 mg once a week (every 7 days) for 11 weeks (Weeks 4 through 14).
Dosage Forms and Strengths
Capsules: 150 mg of oteseconazole (fluconazole is not supplied in the carton). ( 3)
Contraindications
- Females of Reproductive Potential ( 4), ( 5.1), ( 8.3)
- Pregnant and Lactating women ( 4), ( 8.1), ( 8.2)
- Hypersensitivity to oteseconazole ( 4)
Warnings and Precautions
Embryo-Fetal Toxicity: Based on animal studies, VIVJOA may cause fetal harm. The drug exposure window of approximately 690 days (based on 5 times the half-life of oteseconazole) precludes adequate mitigation of the embryo-fetal toxicity risks. Advise patients that VIVJOA is contraindicated in females of reproductive potential, and in pregnant and lactating women because of potential risks to a fetus or breastfed infant. ( 5.1, 8.1, 8.2, 8.3)
Adverse Reactions/Side Effects
The most frequently reported adverse reactions (incidence > 2%) were headache and nausea. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Mycovia Pharmaceuticals, Inc. at 1-855-299-0637 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
BCRP (Breast Cancer Resistance Protein) Substrates: Concomitant use of VIVJOA with BCRP substrates may increase the exposure of drugs that are BCRP substrates, which may increase the risk of adverse reactions associated with these drugs. Use the lowest possible starting dose of the BCRP substrate or consider reducing the dose of the substrate drugs and monitor for adverse reactions. ( 7.1)
Use In Specific Populations
- Renal Impairment: Not recommended in severe renal impairment or ESRD (with or without dialysis). ( 8.6)
- Hepatic Impairment: Not recommended in moderate or severe hepatic impairment. ( 8.7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2022
Related/similar drugs
fluconazole, nystatin topical, clotrimazole topical, Diflucan, itraconazole, miconazole topicalFull Prescribing Information
1. Indications and Usage for Vivjoa
2. Vivjoa Dosage and Administration
2.1 Dosage Overview and Important Administration Instructions
There are two recommended VIVJOA dosage regimens: a VIVJOA-only regimen and a Fluconazole/ VIVJOA regimen. Use one of the following two dosage regimens:
- VIVJOA-only dosage regimen [see Dosage and Administration (2.2)]
- Fluconazole/VIVJOA dosage regimen [see Dosage and Administration (2.3)] .
Administer VIVJOA orally with food [see Clinical Pharmacology (12.3)]. Swallow the capsules whole. Do not chew, crush, dissolve, or open the capsules.
2.2 VIVJOA-only Dosage Regimen
For the VIVJOA-only dosage regimen:
- On Day 1: Administer VIVJOA 600 mg (as a single dose), then
- On Day 2: Administer VIVJOA 450 mg (as a single dose), then
- Beginning on Day 14: Administer VIVJOA 150 mg once a week (every 7 days) for 11 weeks (Weeks 2 through 12).
2.3 Fluconazole/VIVJOA Dosage Regimen
For the Fluconazole/VIVJOA dosage regimen, prescribe fluconazole and:
- On Day 1, Day 4, and Day 7: Administer fluconazole 150 mg orally, then
- On Days 14 through 20: Administer VIVJOA 150 mg once daily for 7 days, then
- Beginning on Day 28: Administer VIVJOA 150 mg once a week (every 7 days) for 11 weeks (Weeks 4 through 14).
3. Dosage Forms and Strengths
VIVJOA Capsules: 150 mg of oteseconazole in lavender hard gelatin capsules imprinted with OTE 150 in black ink.
Fluconazole is not supplied in the carton.
4. Contraindications
VIVJOA is contraindicated in:
- Females of reproductive potential [see Warnings and Precautions (5.1) and Use in Specific Populations (8.3)]
- Pregnant and lactating women [see Warnings and Precautions (5.1), and Use in Specific Populations (8.1, 8.2)]
- Patients with known hypersensitivity to oteseconazole.
5. Warnings and Precautions
5.1 Embryo-Fetal Toxicity
VIVJOA is contraindicated in females of reproductive potential, and in pregnant and lactating women. Based on animal studies, VIVJOA may cause fetal harm. The drug exposure window of approximately 690 days (based on 5 times the half-life of oteseconazole) precludes adequate mitigation of the embryo-fetal toxicity risks. Ocular abnormalities were observed in the offspring of pregnant rats dosed at 7.5-mg/kg/day during organogenesis through lactation in pre and postnatal developmental studies. The observed ocular abnormalities included cataracts, opacities, exophthalmos/buphthalmos, optic nerve/retinal atrophy, lens degeneration and hemorrhage. Ocular abnormalities occurred at doses about 3.5 times the steady state clinical exposure seen with patients being treated for RVVC. Advise patients that VIVJOA is contraindicated in females of reproductive potential, and in pregnant and lactating women because of potential risks to a fetus or breastfed infant [see Use in Specific Populations (8.1, 8.2, 8.3)].
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of one drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 580 patients were treated with VIVJOA in three clinical trials (Trial 1, Trial 2, and Trial 3) [see Clinical Studies (14)] . Patients in the clinical trials were women with RVVC who received VIVJOA treatment for 12 weeks. The mean age of the patient population was 34 years (range:16-78 years), with 84% of patients aged 18-44 years and 16% of patients aged 45 years and older. Although females of reproductive potential were included in the clinical safety data, VIVJOA is contraindicated in females of reproductive potential due to the risk of embryo-fetal toxicity [see Contraindications (4), Warnings and Precautions (5.1), and Use in Specific Populations (8.1, 8.3, 8.4)] .
The clinical trials population was 75% (435/580) White, 17% (96/580) Black or African American, 6% (36/580) Asian, and 2% (13/580) Other women. Fifteen percent (86/580) of all women were Hispanic/Latino. Patients enrolled in the induction and maintenance phases of the clinical trials were treated with different VIVJOA dosage regimens versus comparators [see Clinical Studies (14)] .
The adverse reaction that led to discontinuation in 1 of 580 (0.2 %) VIVJOA-treated patients was allergic dermatitis. Overall, similar percentages of serious adverse reactions and adverse reactions leading to drug discontinuation were reported across the VIVJOA and comparator patient dosing groups.
The most frequently reported adverse reactions (incidence >2%) among VIVJOA-treated patients in Trial 1, Trial 2 and Trial 3 were headache (includes headache, migraines, sinus headaches) (7.4%) and nausea (3.6%).
8. Use In Specific Populations
8.3 Females of Reproductive Potential
VIVJOA is contraindicated in females of reproductive potential based on animal findings. The drug exposure window of approximately 690 days (based on 5 times the half-life of oteseconazole) precludes adequate mitigation of the embryo-fetal toxicity risks [see Warnings and Precautions (5.1), Use in Specific Populations (8.1) and Clinical Pharmacology (12.3)].
Females who are NOT of reproductive potential are defined as: persons who are biological females who are postmenopausal or have another reason for permanent infertility (e.g., tubal ligation, hysterectomy, salpingo-oophorectomy).
8.4 Pediatric Use
VIVJOA is contraindicated in females of reproductive potential. Based on animal studies, VIVJOA may cause fetal harm when administered to a pregnant woman or potential harm to the breastfed infant. The drug exposure window of approximately 690 days (based on 5 times the half-life of oteseconazole) precludes adequate mitigation of the embryo-fetal toxicity risks associated with VIVJOA use [see Contraindications (4), Warnings and Precautions (5.1) and Use in Specific Populations (8.1, 8.2, 8.3) and Clinical Pharmacology (12.3)] .
The safety and effectiveness of VIVJOA have not been established in pre-menarchal pediatric females.
8.5 Geriatric Use
Clinical studies of VIVJOA did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
8.6 Renal Impairment
No dosage adjustment of VIVJOA is recommended in patients with mild to moderate renal impairment (i.e., estimated glomerular filtration rate (eGFR) by the modification of diet in renal disease (MDRD) equation 30-89 mL/min). Clinical studies of VIVJOA did not include sufficient numbers of patients with severe renal impairment (eGFR 15-29 mL/min) or end-stage renal disease (ESRD), defined as eGFR <15 mL/min, to determine the safety of VIVJOA in this population. Therefore, VIVJOA is not recommended for use in patients with severe renal impairment or ESRD (with or without dialysis) [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dosage adjustment of VIVJOA is recommended in patients with mild hepatic impairment (Child-Pugh A). There is insufficient information to determine the safety of VIVJOA in patients with moderate or severe hepatic impairment (Child-Pugh B-C). Therefore, VIVJOA is not recommended for use in patients with moderate or severe hepatic impairment [see Clinical Pharmacology (12.3)].
11. Vivjoa Description
VIVJOA (oteseconazole capsules) contains oteseconazole which is an oral azole antifungal agent.
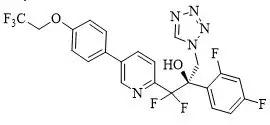
The chemical name of oteseconazole is ( R)-2-(2,4-difluorophenyl)-1,1-difluoro-3-(1 H-tetrazol-1-yl)-1-(5-(4-(2,2,2-trifluoroethoxy)phenyl)pyridin-2-yl)propan-2-ol or 2-Pyridineethanol, α-(2,4-difluorophenyl)-β β-difluoro- α-(1 H-tetrazol-1-ylmethyl)-5-(4-(2,2,2-trifluoroethoxy)phenyl)-,(α R)-. The empirical formula is C 23H 16F 7N 5O 2. The molecular weight is 527.39 g/mol. The structural formula is
Oteseconazole is a white to off-white crystalline powder and is practically insoluble in water within a pH range of 1 to 9 but is soluble in a variety of organic solvents.
Each oteseconazole capsule, for oral use, contains 150 mg oteseconazole and the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, lactose, magnesium stearate, silicified microcrystalline cellulose, and sodium lauryl sulfate. Capsule shell and print constituents: FD&C Blue #1, FD&C Red #3, gelatin, Opacode SW-9008/SW-9009 and titanium dioxide. Contains no ingredient made from a gluten-containing grain (wheat, barley, or rye).
12. Vivjoa - Clinical Pharmacology
12.2 Pharmacodynamics
Oteseconazole exposure-response relationships and the time course of pharmacodynamic response are unknown.
12.3 Pharmacokinetics
The AUC of oteseconazole increased approximately dose proportionally while the C max increased less than dose proportionally over a dose range of 20 mg (0.13 times the lowest recommended dose) to 320 mg (0.53 times the highest recommended dose). The pharmacokinetic parameters of oteseconazole associated with the administration of the recommended dosing regimen of VIVJOA are presented in Table 1.
| PK Parameter * | Mean (± SD) |
|---|---|
|
|
| C max (µg/mL) | 2.8 (1.25) |
| AUC 24h (h∙µg/mL) | 64.2 (29.4) |
| C min (µg/mL) | 2.5 (1.19) |
13. Nonclinical Toxicology
13.2 Animal Toxicology and/or Pharmacology
In an oral carcinogenicity study, Sprague Dawley rats were administered doses of 0.5, 1.5, or 5 mg/kg/day oteseconazole once daily for up to 90 weeks. The high dose was initially reduced from 5 to 3 mg/kg/day in males due to excess mortality. Incidences of hemorrhage were increased in the adrenals, brain, coagulating gland, ears, epididymides, head, heart, lung, nose, pancreas, pharynx, prostate, seminal vesicles, spinal cord, testes, thymus, and bladder of male Crl:CD ®(SD) rats (after 77 weeks of dosing at about 5 times the MRHD based on AUC comparisons). There were no increases in the incidence of hemorrhage in rats after 26 weeks at 5 mg/kg. The clinical relevance of these findings after very high doses (5 to 7 times the MRHD) for the lifetime of the rat remains unclear.
14. Clinical Studies
Trial 1 and Trial 2
Trial 1 and Trial 2 were both randomized, placebo-controlled trials evaluating the efficacy and safety of VIVJOA in the reduction of RVVC. Both trials consisted of two phases: an open-label induction phase and an 11-week maintenance phase. Patients received three sequential doses of 150 mg of fluconazole (every 72 hours) on Days, 1, 4 and 7 during the induction phase. Patients returned 14 days after the first dose of fluconazole and if the acute VVC episode was resolved (vulvovaginal signs and symptoms score < 3) they were randomized (2:1) to receive either 150 mg of VIVJOA or placebo for 7 days followed by 11 weekly doses in the maintenance phase.
In Trial 1, a total of 483 patients were enrolled in the induction phase with 326 patients entering the maintenance phase with 217 patients randomized to VIVJOA and 109 patients randomized to placebo. A total of 182 patients (84%) in the VIVJOA group and 91 patients (83%) in the placebo group completed the trial. The mean age of patients was 34 years old (range 17-78 years old) with 85% of patients aged 18-44 years and 15% of patients aged 45 years and older. Patients were 72% White, 13% Black or African American, 14% Asian, and 8% were of Hispanic or Latino ethnicity.
In Trial 2, a total of 425 patients were enrolled into the induction phase with 330 patients entering the maintenance phase with 220 subjects randomized to VIVJOA and 110 patients randomized to placebo. A total of 191 patients (87%) in the VIVJOA group and 91 patients (83%) in the placebo group completed the trial. The mean age of patients was 34 years old (range 18-73 years old) with 85% of patients aged 18-44 years and 15% of patients aged 45 years and older. Patients were 89% White, 10% Black or African American and 15% were of Hispanic or Latino ethnicity.
For both Trial 1 and Trial 2, efficacy was assessed by the proportion of patients with ≥1 culture-verified acute VVC episode (positive fungal culture for Candida species associated with a clinical signs and symptoms score of ≥3) during the Maintenance Phase through Week 48. Evaluation of clinical signs and symptoms included erythema (redness), edema (swelling), excoriation (skin picking), itching, burning and irritation. Since treatment for acute VVC was allowed to be provided to a patient if it was deemed to be clinically needed when the patient had a signs and symptoms score ≥ 3 and a positive KOH test, the proportion of patients with ≥1 culture-verified acute VVC episode or who took medication known to treat VVC during the Maintenance Phase through Week 48 is also presented.
VIVJOA was superior to placebo with reference to the proportion of patients with ≥1 culture-verified acute VVC episode through Week 48 (Table 2) or the proportion of patients with ≥1 culture-verified acute VVC episode or who took medication known to treat VVC during the Maintenance Phase through Week 48. For both Trial 1 and Trial 2, the average percentage of patients was lower in the VIVJOA groups compared with the placebo group (Table 2).
| Trial 1 | Trial 2 | |||
|---|---|---|---|---|
| VIVJOA
(N=217) | Placebo
(N=109) | VIVJOA
(N=218) | Placebo
(N=108) |
|
| Abbreviations: ITT=Intent-to-Treat (Population); VVC=vulvovaginal candidiasis. | ||||
|
||||
| Proportion of Patients with ≥1 Culture-verified Acute VVC Episode (Day 1 through Week 48) * | 6.7% | 42.8% | 3.9% | 39.4% |
| Treatment Difference p-value † | <0.001 | <0.001 | ||
| Proportion of Patients with ≥1 Culture-verified Acute VVC Episode or received VVC medication (Day 1 through Week 48) * | 27.3% | 50.8% | 21.3% | 49.7% |
| Treatment Difference p-value † | <0.001 | <0.001 | ||
Trial 3
Trial 3 was a randomized, double-blind trial evaluating the efficacy and safety of VIVJOA versus fluconazole and placebo in adults and post-menarchal pediatric females with RVVC. The trial consisted of two phases: induction and maintenance.
During the induction phase, patients received 1050 mg of VIVJOA over two days (600 mg [4×150mg] on Day 1 and 450 mg [3×150mg] on Day 2) or three sequential doses of 150 mg of fluconazole (every 72 hours) on Days, 1, 4 and 7. Patients returned 14 days after the first dose and moved to the maintenance phase if the acute VVC episode was resolved. During the maintenance phase, patients received 150 mg VIVJOA weekly or placebo weekly for 11 weeks.
A total of 219 patients were randomized (2:1) into the induction phase: 147 to VIVJOA and 72 to fluconazole/placebo. One patient in the VIVJOA group did not receive drug therefore 146 patients received VIVJOA. A total of 112 patients (76%) in the VIVJOA group and 55 patients (76%) in the fluconazole/placebo group completed the trial.
The mean age of patients was 35 years (range 16-78) with 80% of patients aged 18-44 years and 19% of patients aged 45 years and older. Patients were 59% White, 34% Black or African American, 1% Asian and 26% were of Hispanic or Latino ethnicity. The trial was conducted completely in the United States.
Efficacy was assessed by the proportion of patients with ≥1 culture verified acute VVC episode during the maintenance phase (post-randomization through Week 50) or who failed clearing their infection during the induction phase. A recurring acute VVC episode was defined as a positive culture for Candida species and a clinical signs and symptoms score of ≥3. Evaluation of clinical signs and symptoms included erythema(redness), edema (swelling), excoriation (skin picking), itching, burning and irritation. Additionally, the proportion of patients with ≥1 culture verified acute VVC episode or who took medication known to treat VVC during the maintenance phase (post-randomization through Week 50) or who failed clearing their infection during the induction phase is presented.
VIVJOA was superior to fluconazole/placebo in the proportion of patients with ≥1 culture-verified recurring acute VVC episode during the maintenance phase (post randomization through Week 50) or failed clearing their infection during the induction phase and the proportion of patients with ≥1 culture-verified recurring acute VVC episode or took VVC medication known to treat VVC during the maintenance phase (post randomization through Week 50) or who failed clearing their infection during the induction phase. The average percentage of patients was lower in the VIVJOA group compared with the fluconazole/placebo group (Table 3).
| VIVJOA
(N=147) | Fluconazole/Placebo
(N=72) | Treatment Difference p-value * | |
|---|---|---|---|
| Abbreviations: ITT=Intent-to-Treat (Population); VVC=vulvovaginal candidiasis | |||
|
|||
| Proportion of Patients with ≥1 Culture-verified Acute VVC Episode through Week 50 or Unresolved VVC Episode During the Induction Phase † | 10.3% | 42.9% | <0.001 |
| Proportion of Patients with ≥1 Culture-verified Acute VVC Episode or took VVC medication through Week 50 or Unresolved VVC Episode During the Induction Phase † | 43.5% | 59.0% | 0.039 |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
| VIVJOA
oteseconazole capsule |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Mycovia Pharmaceuticals, Inc. (079575708) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon, Inc. (a ThermoFisher Company) | 205475333 | manufacture(74695-823) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon, Inc. | 240769596 | analysis(74695-823) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Packaging Coordinators, LLC. | 078525133 | pack(74695-823) , label(74695-823) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Evonik Corporation Tippecanoe Laboratories | 130890994 | api manufacture(74695-823) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Micro-macinazione SA Lonza Pharm and Biotech | 480918515 | particle size reduction(74695-823) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dynamit Nobel GmBH Explosivoff-und Systemtechnik (Novasep) | 313113144 | api manufacture(74695-823) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ChemCon | 328823245 | analysis(74695-823) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Currenta GmBH &Co OHG | 331575303 | analysis(74695-823) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioChem Labor fur biologische und chemische Analytik GmBH | 318354230 | analysis(74695-823) | |