Drug Detail:Xadago (Safinamide [ sa-fin-a-mide ])
Drug Class: Dopaminergic antiparkinsonism agents
Highlights of Prescribing Information
XADAGO ®
(safinamide) tablets
For Oral Use.
Initial U.S. Approval: 2017
Indications and Usage for Xadago
XADAGO is a monoamine oxidase type B (MAO-B) inhibitor indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson's disease (PD) experiencing "off" episodes ( 1)
Limitations of Use: XADAGO has not been shown to be effective as monotherapy for the treatment of PD.
Xadago Dosage and Administration
- Start with 50 mg administered orally once daily at the same time of day; after two weeks, the dose may be increased to 100 mg once daily, based on individual need and tolerability ( 2.1)
- Hepatic Impairment: Do not exceed 50 mg once daily in patients with moderate hepatic impairment; contraindicated in patients with severe hepatic impairment ( 2.2, 4)
Dosage Forms and Strengths
Tablets: 50 mg and 100 mg ( 3)
Contraindications
XADAGO is contraindicated in patients with:
- Concomitant use of the following drugs:
- Other monoamine oxidase inhibitors or other drugs that are potent inhibitors of monoamine oxidase (e.g., linezolid) ( 4, 7.1)
- Opioid drugs (e.g., tramadol, meperidine and related derivatives); selective norepinephrine reuptake inhibitors; tri-or tetra-cyclic or triazolopyridine antidepressants; cyclobenzaprine; methylphenidate, amphetamine, and their derivatives; St. John's wort ( 4, 7.2, 7.3, 7.5)
- Dextromethorphan ( 4, 7.4)
- A history of a hypersensitivity to safinamide ( 4)
- Severe hepatic impairment (Child-Pugh C: 10-15) ( 4)
Warnings and Precautions
- May cause or exacerbate hypertension ( 5.1)
- May cause serotonin syndrome when used with MAO inhibitors, antidepressants, or opioid drugs ( 5.2)
- May cause falling asleep during activities of daily living ( 5.3)
- May cause or exacerbate dyskinesia; consider levodopa dose reduction ( 5.4)
- May cause hallucinations and psychotic behavior ( 5.5)
- May cause problems with impulse control/compulsive behaviors ( 5.6)
- May cause withdrawal-emergent hyperpyrexia and confusion ( 5.7)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence on XADAGO 100 mg/day at least 2% greater than placebo) were dyskinesia, fall, nausea, and insomnia ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact US WorldMeds, LLC, at 1-888-492-3246 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Selective Serotonin Reuptake Inhibitors: Monitor patients for serotonin syndrome ( 7.3)
- Sympathomimetic Medications: Monitor patients for hypertension ( 7.5)
- Tyramine: Risk of severe hypertension ( 7.6)
- Substrates of Breast Cancer Resistance Protein (BCRP): Potential increase in plasma concentration of BCRP substrate ( 7.7)
Use In Specific Populations
Pregnancy: Based on animal data, may cause fetal harm ( 8.1).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2017
Related/similar drugs
ropinirole, pramipexole, carbidopa / levodopa, benztropine, Exelon, GocovriFull Prescribing Information
1. Indications and Usage for Xadago
XADAGO is indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson's disease (PD) experiencing "off" episodes.
2. Xadago Dosage and Administration
2.1 Dosing Information
The recommended starting dosage of XADAGO is 50 mg administered orally once daily (at the same time of day), without regard to meals. After two weeks, the dosage may be increased to 100 mg once daily, based on individual need and tolerability.
Daily dosages of XADAGO above 100 mg have not been shown to provide additional benefit, and higher dosages increase the risk for adverse reactions. XADAGO has been shown to be effective only in combination with levodopa/carbidopa [see Indications and Usage (1)] .
If a dose is missed, the next dose should be taken at the same time the next day.
XADAGO 100 mg should be tapered by decreasing the dose to 50 mg for one week before stopping [see Warnings and Precautions (5.7)] .
2.2 Dosing in Patients with Hepatic Impairment
In patients with moderate hepatic impairment (Child-Pugh B: 7-9), the maximum recommended dosage of XADAGO is 50 mg orally once daily. XADAGO is contraindicated in patients with severe hepatic impairment (Child-Pugh C: 10-15) [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)] . If a patient taking 50 mg XADAGO progresses from moderate to severe hepatic impairment, discontinue XADAGO.
3. Dosage Forms and Strengths
- 50 mg tablets: Orange to copper with metallic gloss, round, biconcave shaped embossed with "50" on one side
- 100 mg tablets: Orange to copper with metallic gloss, round, biconcave shaped embossed with "100" on one side
4. Contraindications
XADAGO is contraindicated in patients with:
- Concomitant use of other drugs in the monoamine oxidase inhibitor (MAOI) class or other drugs that are potent inhibitors of monoamine oxidase, including linezolid. The combination may result in increased blood pressure, including hypertensive crisis [see Warnings and Precautions (5.1) and Drug Interactions (7.1)] .
- Concomitant use of opioid drugs (e.g., meperidine and its derivatives, methadone, propoxyphene, or tramadol); serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic, tetracyclic, or triazolopyridine antidepressants; cyclobenzaprine; methylphenidate, amphetamine, and their derivatives; or St John's wort. Concomitant use could result in life-threatening serotonin syndrome [see Warnings and Precautions (5.2) and Drug Interactions (7.2, 7.3, 7.5)] .
- Concomitant use of dextromethorphan. The combination of MAO inhibitors and dextromethorphan has been reported to cause episodes of psychosis or abnormal behavior [see Drug Interactions (7.4)] .
- A history of a hypersensitivity to safinamide. Reactions have included swelling of the tongue and oral mucosa, and dyspnea.
- Severe hepatic impairment (Child-Pugh C: 10-15) [see Use in Specific Populations (8.6)] .
5. Warnings and Precautions
5.1 Hypertension
XADAGO may cause hypertension or exacerbate existing hypertension. In clinical trials, the incidence of hypertension was 7% for XADAGO 50 mg, 5% for XADAGO 100 mg, and 4% for placebo. Monitor patients for new onset hypertension or hypertension that is not adequately controlled after starting XADAGO. Medication adjustment may be necessary if elevation of blood pressure is sustained.
Monitor for hypertension if XADAGO is prescribed concomitantly with sympathomimetic medications, including prescription or nonprescription nasal, oral, and ophthalmic decongestants and cold remedies [see Drug Interactions (7.5)] .
XADAGO is a selective inhibitor of MAO-B at the recommended dosages of 50 mg or 100 mg daily. Selectivity for inhibiting MAO-B decreases above the recommended daily dosages [see Clinical Pharmacology (12.2)] . Therefore, XADAGO should not be used at daily dosages exceeding those recommended because of the risks of hypertension, exacerbation of existing hypertension, or hypertensive crisis.
Dietary tyramine restriction is not required during treatment with recommended doses of XADAGO. However, use with certain foods that contain very high amounts (i.e., more than 150 mg) of tyramine could cause severe hypertension, resulting from an increased sensitivity to tyramine in patients taking recommended dosages of XADAGO, and patients should be advised to avoid such foods.
Isoniazid has some monoamine oxidase inhibiting activity. Monitor for hypertension and reaction to dietary tyramine in patients treated concomitantly with isoniazid and XADAGO [see Drug Interactions (7.1, 7.6)] .
5.2 Serotonin Syndrome
The development of a potentially life-threatening serotonin syndrome has been reported in patients on concomitant treatment with MAO inhibitors (including selective MAO-B inhibitors), serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants, tetracyclic antidepressants, triazolopyridine antidepressants, cyclobenzaprine, opioid drugs (e.g., meperidine and meperidine derivatives, propoxyphene, tramadol), and methylphenidate, amphetamine, and their derivatives. Concomitant use of XADAGO with these drugs is contraindicated.
In clinical trials, serotonin syndrome was reported in a patient treated with XADAGO and an SSRI. Use the lowest effective dose of SSRIs in patients treated with concomitant XADAGO.
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
5.3 Falling Asleep During Activities of Daily Living
Patients treated with dopaminergic medications have reported falling asleep while engaged in activities of daily living, including the operation of motor vehicles, which sometimes has resulted in accidents. Patients may not perceive warning signs, such as excessive drowsiness, or they may report feeling alert immediately prior to the event.
In clinical studies, sleep attacks/sudden onset of sleep were reported in patients treated with XADAGO 100 mg/day.
If a patient develops daytime sleepiness or episodes of falling asleep during activities that require full attention (e.g., driving a motor vehicle, conversations, eating), XADAGO should ordinarily be discontinued. If a decision is made to continue these patients on XADAGO, advise them to avoid driving and other potentially dangerous activities.
5.4 Dyskinesia
XADAGO may cause dyskinesia or exacerbate pre-existing dyskinesia.
In clinical trials, the incidence of dyskinesia was 21% for XADAGO 50 mg, 18% for XADAGO 100 mg, and 9% for placebo. There was a greater incidence of dyskinesia causing study discontinuation in Parkinson's disease patients treated with XADAGO 50 mg or 100 mg (1%), compared to placebo (0%) [see Adverse Reactions (6.1)] .
Reducing the patient's daily levodopa dosage or the dosage of another dopaminergic drug may mitigate dyskinesia.
5.5 Hallucinations / Psychotic Behavior
Patients with a major psychotic disorder should ordinarily not be treated with XADAGO because of the risk of exacerbating the psychosis with an increase in central dopaminergic tone. In addition, treatments for psychosis that antagonize the effects of dopaminergic medications may exacerbate the symptoms of Parkinson's disease [see Drug Interactions (7.8)] .
Consider dosage reduction or stopping the medication if a patient develops hallucinations or psychotic-like behaviors while taking XADAGO.
5.6 Impulse Control / Compulsive Behaviors
Patients can experience intense urges to gamble, increased sexual urges, intense urges to spend money, binge eating, and/or other intense urges, and the inability to control these urges while taking one or more of the medications, including XADAGO, that increase central dopaminergic tone. In some cases, these urges were reported to have stopped when the dose was reduced or the medication was discontinued. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending or other urges while being treated with XADAGO. Consider dose reduction or stopping the medication if a patient develops such urges while taking XADAGO.
5.7 Withdrawal-Emergent Hyperpyrexia and Confusion
A symptom complex resembling neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in drugs that increase central dopaminergic tone.
5.8 Retinal Pathology
Retinal degeneration and loss of photoreceptor cells were observed in albino and pigmented rats administered safinamide orally in toxicity studies of up to 6 months duration. In albino rats administered safinamide orally for two years, retinal scarring and cataracts were observed at all doses tested [see Nonclinical Toxicology (13.2)] .
Periodically monitor patients for visual changes in patients with a history of retinal/macular degeneration, uveitis, inherited retinal conditions, family history of hereditary retinal disease, albinism, retinitis pigmentosa, or any active retinopathy (e.g., diabetic retinopathy).
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed in greater detail in other sections of labeling:
- Hypertension [see Warnings and Precautions (5.1)]
- Serotonin Syndrome [see Warnings and Precautions (5.2)]
- Falling Asleep During Activities of Daily Living [see Warnings and Precautions (5.3)]
- Dyskinesia [see Warnings and Precautions (5.4)]
- Hallucinations / Psychotic Behavior [see Warnings and Precautions (5.5)]
- Impulse Control / Compulsive Behaviors [see Warnings and Precautions (5.6)]
- Withdrawal-Emergent Hyperpyrexia and Confusion [see Warnings and Precautions (5.7)]
- Retinal Pathology [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Clinical trials are conducted under widely varying conditions; therefore, adverse reactions observed in the clinical trials of a drug cannot be directly compared to the incidence in the clinical trials of another drug and may not reflect the incidence observed in clinical practice.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval of use of safinamide outside of the United States. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
A postmarketing report describes a patient who developed a hypersensitivity reaction consisting of swelling of the tongue and gingiva, dyspnea and skin rash. The symptoms resolved shortly after XADAGO was discontinued, but reappeared following rechallenge a month later.
7. Drug Interactions
7.1 MAO Inhibitors
XADAGO is contraindicated for use with other drugs in the MAO inhibitor class or other drugs that are potent inhibitors of monoamine oxidase (e.g., linezolid, an oxazolidinone antibacterial, which also has reversible nonselective MAO inhibition activity). Co-administration increases the risk of nonselective MAO inhibition, which may lead to hypertensive crisis [see Contraindications (4) and Warnings and Precautions (5.1)] . At least 14 days should elapse between discontinuation of XADAGO and initiation of treatment with other MAOIs.
Isoniazid has some monoamine oxidase inhibiting activity. Monitor for hypertension and reaction to dietary tyramine in patients treated concomitantly with isoniazid and XADAGO.
7.2 Opioid Drugs
Because serious, sometimes fatal reactions have been precipitated with concomitant use of XADAGO with opioid drugs (e.g., meperidine and its derivatives, methadone, propoxyphene, or tramadol) and MAO inhibitors, including selective MAO-B inhibitors, concomitant use of these drugs is contraindicated [see Warnings and Precautions (5.2)] . At least 14 days should elapse between discontinuation of XADAGO and initiation of treatment with these drugs.
7.3 Serotonergic Drugs
Concomitant use of XADAGO with SNRIs; triazolopyridine, tricyclic or tetracyclic antidepressants; cyclobenzaprine (a skeletal muscle relaxant that is a tricyclic antidepressant derivative); or St. John's wort is contraindicated [see Warnings and Precautions (5.2)] . At least 14 days should elapse between discontinuation of XADAGO and initiation of treatment with these drugs.
Monitor patients for symptoms of serotonin syndrome if selective serotonin re-uptake inhibitors are used by patients treated with XADAGO [see Warnings and Precautions (5.2)] .
7.4 Dextromethorphan
The combination of MAO inhibitors and dextromethorphan has been reported to cause episodes of psychosis or bizarre behavior. Therefore, in view of XADAGO's MAO inhibitory activity, dextromethorphan is contraindicated for use with XADAGO.
7.5 Sympathomimetic Medications
Severe hypertensive reactions have followed the administration of sympathomimetics and nonselective MAO inhibitors. Hypertensive crisis has been reported in patients taking the recommended doses of selective MAO-B inhibitors and sympathomimetic medications. Concomitant use of XADAGO with methylphenidate, amphetamine, and their derivatives is contraindicated [see Warnings and Precautions (5.1, 5.2)] .
Monitor patients for hypertension if XADAGO is prescribed concomitantly with prescription or nonprescription sympathomimetic medications, including nasal, oral, or ophthalmic decongestants and cold remedies [see Warnings and Precautions (5.1)] .
7.6 Tyramine
MAO in the gastrointestinal tract and liver (primarily type A) provides protection from exogenous amines (e.g., tyramine). If tyramine were absorbed intact, it could lead to severe hypertension, including hypertensive crisis. Aged, fermented, cured, smoked, and pickled foods containing large amounts of exogenous amines (e.g., aged cheese, pickled herring) may cause release of norepinephrine resulting in a rise in blood pressure (Tyramine Reaction). Patients should be advised to avoid foods containing a large amount of tyramine while taking recommended doses of XADAGO [see Warnings and Precautions (5.1)] .
Selectivity for inhibiting MAO-B decreases in a dose-related manner above the highest recommended daily dosage, which may increase the risk for hypertension [see Clinical Pharmacology (12.2)] . In addition, isoniazid has some monoamine oxidase inhibiting activity. Monitor for hypertension and reaction to dietary tyramine in patients treated with isoniazid and XADAGO [see Warnings and Precautions (5.1)] .
7. 7 Substrates of Breast Cancer Resistance Protein (BCRP)
Safinamide and its major metabolite may inhibit intestinal breast cancer resistance protein (BCRP) [see Clinical Pharmacology (12.3)] . Inhibition of BCRP could increase plasma concentrations of BCRP substrates. Examples of substrates of BCRP include methotrexate, mitoxantrone, imatinib, irrinotecan, lapatinib, rosuvastatin, sulfasalazine, and topotecan. Monitor patients for increased pharmacologic or adverse effect of the BCRP substrates if XADAGO is used concomitantly.
8. Use In Specific Populations
8.3 Nursing Mothers
Skin discoloration, presumed to be caused by hyperbilirubinemia resulting from hepatobiliary toxicity, was observed in rat pups indirectly exposed to safinamide through the milk during the lactation period. It is not known whether this drug is present in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from safinamide, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and effectiveness of XADAGO in pediatric patients have not been established. No juvenile toxicity studies have been performed in animals.
8.5 Geriatric Use
Of the 1516 subjects exposed to XADAGO in clinical studies, 38% were 65 and over, while 4% were 75 and over. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Hepatic Impairment
XADAGO plasma concentrations are increased in patients with hepatic impairment [see Clinical Pharmacology (12.3)] .
In patients with moderate hepatic impairment (Child-Pugh B: 7-9), the maximum recommended dosage of XADAGO is 50 mg once daily [see Dosage and Administration (2.2)] . XADAGO has not been studied in patients with severe hepatic impairment (Child-Pugh C: 10-15), and is contraindicated in these patients. If patients progress from moderate to severe hepatic impairment, treatment with XADAGO should be stopped.
10. Overdosage
There is no human experience with XADAGO overdose.
There is no known antidote to XADAGO nor any specific treatment for XADAGO overdose. If an important overdose occurs, XADAGO treatment should be discontinued and supportive treatment should be administered as clinically indicated. In cases of overdose with XADAGO, dietary tyramine restriction should be observed for several weeks.
The Poison Control Center should be called at 1-800-222-1222 for the most current treatment guidelines.
11. Xadago Description
XADAGO tablets contain safinamide, which is a MAO-B inhibitor, as the mesylate salt. Safinamide mesylate is (S)-2- [[4-[(3-fluorophenyl) methoxy]phenyl]methyl]aminopropanamide methanesulfonate (1:1) and its structural formula is below.
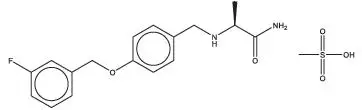
The molecular formula of safinamide mesylate is C 17H 19FN 2O 2∙CH 4O 3S and its molecular weight is 398.45.
Safinamide mesylate is a white to off-white crystalline powder. Safinamide mesylate is freely soluble in water, methanol and dimethyl sulfoxide. Safinamide mesylate is sparingly soluble in ethanol and is practically insoluble in ethyl acetate. In aqueous buffers that span a pH range of 1.2 to 7.5, safinamide mesylate is highly soluble at pH 1.2 and 4.5, but shows low solubility (<0.4 mg/mL) at pH 6.8 and 7.5.
XADAGO is available as 50 mg and 100 mg film-coated tablets for oral administration. Each XADAGO tablet contains 65.88 mg or 131.76 mg of safinamide mesylate, equivalent to 50 mg or 100 mg, respectively, of safinamide free base. The tablets also contain the following inactive ingredients: colloidal silicon dioxide, crospovidone, hypromellose, iron oxide (red), magnesium stearate, microcrystalline cellulose, polyethylene glycol 6000, potassium aluminum silicate, and titanium dioxide.
12. Xadago - Clinical Pharmacology
12.1 Mechanism of Action
The precise mechanism by which XADAGO exerts its effect in Parkinson's disease is unknown. XADAGO is an inhibitor of monoamine oxidase B (MAO-B). Inhibition of MAO-B activity, by blocking the catabolism of dopamine, is thought to result in an increase in dopamine levels and a subsequent increase in dopaminergic activity in the brain.
12.2 Pharmacodynamics
XADAGO inhibits monoamine oxidase B (MAO-B), with more than 1000-fold selectivity over MAO-A. In clinical studies, complete inhibition (>90%) of MAO-B was measured at doses > 20 mg.
13. Nonclinical Toxicology
14. Clinical Studies
14.1 Adjunctive Treatment in Patients with Parkinson's Disease Experiencing OFF Time on a Stable Dose of Levodopa.
Two double-blind, placebo-controlled, multi-national, 24-week studies (Study 1 and Study 2) were conducted in PD patients experiencing "OFF" Time during treatment with carbidopa/levodopa and other PD medications, e.g., dopamine agonists, catechol-O-methyl transferase (COMT) inhibitors, anticholinergics, and/or amantadine. In both studies, the primary measure of effectiveness was the change from baseline in total daily "ON" Time without troublesome dyskinesia (i.e., "ON" Time without dyskinesia plus "ON" Time with non- troublesome dyskinesia), based on 18-hour diaries completed by patients for at least 3 days before each of the scheduled visits. Secondary endpoints included "OFF" Time during the diary period and reduction in Uniform Parkinson's Disease Rating Scale (UPDRS) Part III (motor examination).
In Study 1, patients (n=645) were randomized equally to treatment with XADAGO 50 mg/day (n=217 patients), XADAGO 100 mg/day (n=216 patients), or placebo (n=212 patients), and had at least one post-baseline assessment of "ON" Time.
The percentages of patients taking stable doses of other classes of PD medications, in addition to levodopa/decarboxylase inhibitor, were: dopamine agonists (61%), COMT inhibitors (24%), anticholinergics (37%), and amantadine (14%). Use of MAO inhibitors was prohibited. The average daily dosage of levodopa was 630 mg. The mean duration of Parkinson's disease was approximately 8 years.
In Study 1, XADAGO 50 mg/day and 100 mg/day significantly increased "ON" Time compared to placebo (Table 2). The increase in "ON" Time without troublesome dyskinesia was accompanied by a similar significant reduction in "OFF" Time and a reduction in Unified Parkinson's Disease Rating Scale Part III (UPDRS III) scores assessed during "ON" Time (Table 3). Improvement in "ON Time" occurred without an increase in troublesome dyskinesia.
| N | Baseline (hours)
(mean ± SD) | Change from Baseline to Endpoint
(LSD † vs. placebo) (95% CI) ‡ p-value |
|
|---|---|---|---|
|
|||
| Placebo | 212 | 9.3 ± 2.2 | -- |
| XADAGO 50 mg once daily | 217 | 9.4 ± 2.2 | 0.50 (0.03, 0.96)
p=0.0356 |
| XADAGO 100 mg once daily | 216 | 9.6 ± 2.5 | 0.53 (0.07, 1.00)
p=0.0238 |
| N | Baseline (hours)
(mean ± SD) | Change from Baseline to Endpoint
(LSD * vs. placebo) (95% CI) † p-value |
|
|---|---|---|---|
|
|||
| Change in mean daily "OFF" time | |||
| Placebo | 212 | 5.3 ± 2.1 | -- |
| XADAGO 50 mg once daily | 217 | 5.2 ± 2.0 | -0.55 (-0.93, -0.17) p=0.0049 |
| XADAGO 100 mg once daily | 216 | 5.2 ± 2.2 | -0.57 (-0.95, -0.19) p=0.0037 |
| Change in UPDRS Part III (Motor subscale) | |||
| Placebo | 212 | 28.6 ± 12.0 | -- |
| XADAGO 50 mg once daily | 217 | 27.3 ± 12.8 | -1.75 (-3.24, -0.36) p=0.0212 |
| XADAGO 100 mg once daily | 216 | 28.4 ± 13.5 | -2.48 (-3.97, -1.00) p=0.0011 |
The effect of XADAGO 100 mg on "ON" Time was only slightly numerically greater than the effect of XADAGO 50 mg. In addition, the time course of improvement in total daily "ON" Time was similar between both doses (Figure 1). The time course of improvement in total daily "ON" Time showed numerically greater improvement with both XADAGO 50mg and 100 mg compared to placebo, at all post-baseline timepoints (Figure 1).
Figure 1: Mean of Change from Baseline in Total Daily "ON" Time by Week and Treatment in Study 1
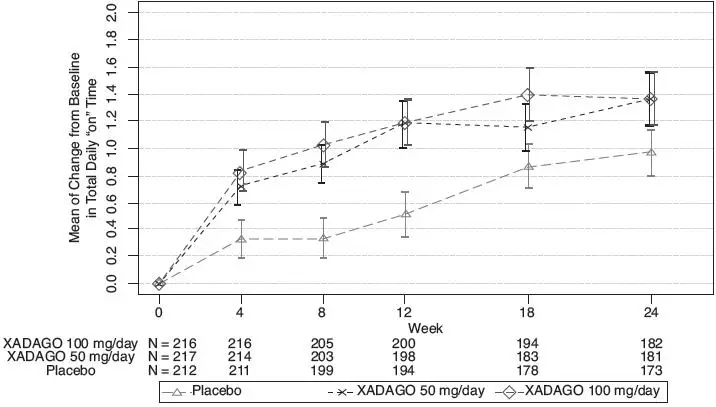
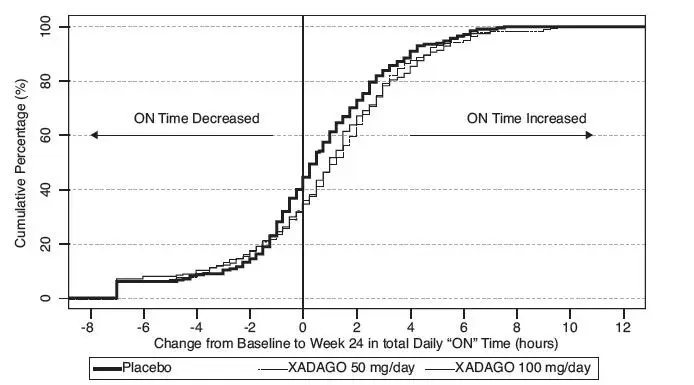
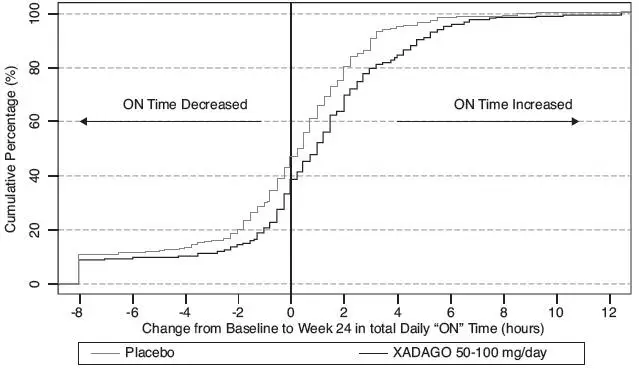
Figure 2 shows the empirical cumulative distribution functions (CDF) for the change from baseline to Week 24 in total daily "ON" Time in Study 1. The cumulative percentage of patients with a change in "ON" Time was similar for the XADAGO 50 mg and 100 mg groups. The cumulative percentage of patients with an increase in "ON" Time is higher for both XADAGO 50 mg and 100 mg treated patients than for placebo patients.
Figure 2: Study 1 Empirical Cumulative Distribution Functions (CDF) for the Change from Baseline to Week 24 in Total Daily "ON" Time
Patients who dropped out of the study because of an adverse reaction, lack of efficacy, non-compliance, or withdrawal of consent were treated as treatment failures and assumed to have the smallest change from baseline among all patients. The failure rates are 6.1%, 5.6%, and 6.9% for the placebo group, XADAGO 50 mg/day group, and XADAGO 100 mg/day group, respectively.
In Study 2, patients (n=549) were randomized to treatment with XADAGO 100 mg daily (n=274 patients) or placebo (n=275 patients) for up to 24 weeks. The percentages of patients taking stable doses of other classes of PD medication, in addition to levodopa/decarboxylase inhibitor, were: dopamine agonists (74%), COMT inhibitors (18%), anticholinergics (17%), and amantadine (30%). Use of MAO inhibitors was prohibited. The average daily dosage of levodopa was 777 mg. The mean duration of Parkinson's disease was approximately 9 years.
In Study 2, XADAGO was significantly better than placebo for increasing "ON" Time (Table 4). The observed increase in "ON" Time without troublesome dyskinesia was accompanied by a reduction in "OFF" Time of similar magnitude and a reduction in UPDRS III score (assessed during "ON" Time). The time course of effect was similar to that showed in the above figure for Study 1. As in Study 1, the increase in "ON" Time without troublesome dyskinesia was accompanied by a similar significant reduction in "OFF" Time and a reduction in Unified Parkinson's Disease Rating Scale Part III (UPDRS III) scores assessed during "ON" Time (Table 5).
| N | Baseline (hours)
(mean ± SD) | Change from Baseline to Endpoint
(LSD † vs. placebo) (95% CI) ‡ | p-value | |
|---|---|---|---|---|
|
||||
| Placebo | 273 | 9.1 ± 2.5 | -- | -- |
| XADAGO 100 mg once daily | 270 | 9.3 ± 2.4 | 0.99 (0.58, 1.39) | < 0.001 |
| N | Baseline (hours)
(mean ± SD) | Change from Baseline to Endpoint
(LSD * vs. placebo) (95% CI) † p-value |
|
|---|---|---|---|
|
|||
| Change in mean daily "OFF" time | |||
| Placebo | 273 | 5.36 ± 2.00 | -- |
| XADAGO 100 mg once daily | 270 | 5.35 ± 1.98 | -1.06 (-1.43, -0.69)
<0.001 |
| Change in UPDRS Part III (Motor subscale) | |||
| Placebo | 273 | 23.03 ± 12.75 | -- |
| XADAGO 100 mg once daily | 270 | 22.33 ± 11.79 | -1.70 (-2.89, -0.50)
0.005 |
The time course of improvement in total daily "ON" Time showed numerically greater improvement with XADAGO 100 mg compared to placebo at all post-baseline timepoints (Figure 3).
Figure 3: Mean of Change from Baseline in Total Daily "ON" Time by Week and Treatment in Study 2
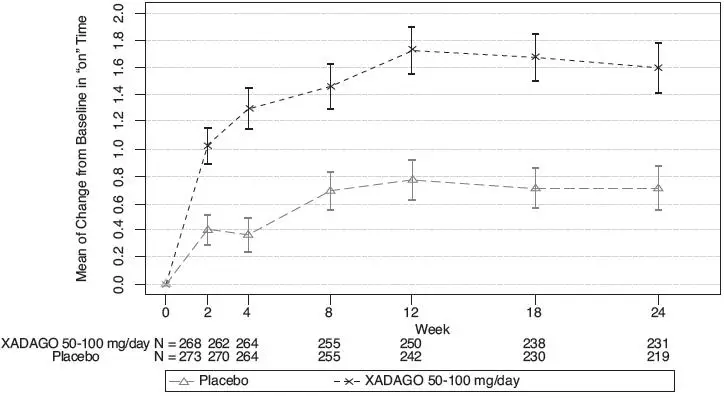
Figure 4 shows the empirical cumulative distribution functions (CDF) for the change from baseline to Week 24 in total daily "ON" Time in Study 2. The cumulative percentage of patients with an increase in "ON" Time treated with XADAGO 50 mg to 100 mg is higher than for placebo patients.
Figure 4: Study 2 Empirical Cumulative Distribution Functions (CDF) for the Change from Baseline to Week 24 in Total Daily "ON" Time
16. How is Xadago supplied
16.1 How Supplied
50 mg (orange to copper colored with metallic gloss, round film-coated, biconcave shaped tablet embossed with "50" on one side; approximately 7 mm in diameter).
| Bottles of 30 tablets | NDC 27505-110-30 |
| Bottles of 90 tablets | NDC 27505-110-90 |
100 mg (orange to copper colored with metallic gloss, round film-coated, biconcave shaped tablet embossed with "100" on one side; approximately 9 mm in diameter).
| Bottles of 30 tablets | NDC 27505-111-30 |
| Bottles of 90 tablets | NDC 27505-111-90 |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
PRINCIPAL DISPLAY PANEL - 50 mg Tablet Bottle Label
Bottle Label
Rx only
NDC 27505- 110-30
Xadago ®
(safinamide) tablets
50 mg
Keep out of reach of children.
30 tablets
US WorldMeds™
PRINCIPAL DISPLAY PANEL - 50mg Shipper Label
SHIPPING LABEL
Product: Xadago 50mg OP 30 USA
Label Item Number: PCD4454785ZAMBON
Label Version Number: 454785-V001
Batch No:
Expiry Date:
Item-no.:
Outer box No.:
Number of finished packs per outer: 24
SHIPPED TO:
ZAMBON
SHIPPED FROM:
Catalent Germany Schorndorf GmBH

PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle Carton
Rx only
NDC 27505-
111-30
Xadago
®
(safinamide) tablets
100 mg
Keep out of reach of children.
30 tablets
US WorldMeds™

| XADAGO
safinamide mesylate tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| XADAGO
safinamide mesylate tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Catalent Germany Schorndorf GmbH (315732628) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent Germany Schorndorf GmbH | 315732628 | manufacture(68621-0030, 68621-0031) , pack(68621-0030, 68621-0031) , label(68621-0030, 68621-0031) | |







