Drug Detail:Xepi (Ozenoxacin topical [ oz-en-ox-a-sin-top-i-kal ])
Drug Class: Topical antibiotics
Highlights of Prescribing Information
XEPI™ (ozenoxacin) cream, for topical use
Initial U.S. Approval: 2017
Indications and Usage for Xepi Cream
XEPI is a quinolone antimicrobial indicated for the topical treatment of impetigo due to Staphylococcus aureus or Streptococcus pyogenes in adult and pediatric patients 2 months of age and older (1).
Xepi Cream Dosage and Administration
- Apply a thin layer of XEPI topically to the affected area twice daily for 5 days (2).
- Affected area may be up to 100 cm2 in adult and pediatric patients 12 years of age and older or 2% of the total body surface area and not exceeding 100 cm2 in pediatric patients less than 12 years of age (2).
- For topical use only (2).
- Not for oral, ophthalmic, intranasal, or intravaginal use (2).
Dosage Forms and Strengths
Cream: Each gram contains 10 mg of ozenoxacin (1%) (3).
Contraindications
None (4)
Warnings and Precautions
Potential for Microbial Overgrowth: Prolonged use of XEPI may result in overgrowth of nonsusceptible bacteria and fungi. If such infections occur, discontinue use and institute alternative therapy (5).
Adverse Reactions/Side Effects
Adverse reactions (rosacea and seborrheic dermatitis) were reported in 1 adult patient treated with XEPI (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Biofrontera, Inc. at 1-844-829-7434 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2020
Related/similar drugs
cefuroxime, mupirocin topical, cefadroxil, Bactroban, Ceftin, DuricefFull Prescribing Information
1. Indications and Usage for Xepi Cream
XEPI™ is indicated for the topical treatment of impetigo due to Staphylococcus aureus or Streptococcus pyogenes in adult and pediatric patients 2 months of age and older [see Clinical Studies (14)].
2. Xepi Cream Dosage and Administration
Apply a thin layer of XEPI topically to the affected area twice daily for five days. Affected area may be up to 100 cm2 in adult and pediatric patients 12 years of age and older or 2% of the total body surface area and not exceeding 100 cm2 in pediatric patients less than 12 years of age.
- Wash hands after applying XEPI cream.
- XEPI cream is for topical use only.
- Not for oral, ophthalmic, intranasal, or intravaginal use.
- The treated area may be covered with a sterile bandage or gauze dressing.
3. Dosage Forms and Strengths
Cream: 1%, pale yellow cream. Each gram of XEPI contains 10 mg of ozenoxacin.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety profile of XEPI was assessed in two clinical trials (Trial 1 and Trial 2) in 362 adult and pediatric patients two months of age and older with impetigo. The patients used at least one dose from a 5-day, twice a day regimen of XEPI. Control groups included 361 patients who used placebo and 152 patients who used retapamulin ointment. The median age of the patients enrolled in the clinical trials was 10 years; 3 % of patients were 2 months to less than 2 years of age, 55 % of patients were 2 to less than 12 years of age, 11 % of patients were 12 to less than 18 years of age, and 31 % of patients were 18 years of age or older.
Adverse reactions (rosacea and seborrheic dermatitis) were reported in 1 adult patient treated with XEPI.
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of XEPI in the treatment of impetigo have been established in pediatric patients 2 months to 17 years of age. Use of XEPI in pediatric patients (2 months to 17 years of age) is supported by evidence from adequate and well-controlled studies of XEPI in which 251 pediatric patients received at least one dose of XEPI. The median age of the patients enrolled in the clinical trials was 10 years; 3 % of patients were 2 months to less than 2 years of age, 55 % of patients were 2 to less than 12 years of age, 11 % of patients were 12 to less than 18 years of age, and 31 % of patients were 18 years of age or older. In these studies, the maximum dose applied was approximately 0.5 g of XEPI applied twice daily for up to 5 days (i.e., up to 10 applications total) [see Clinical Studies (14)].
The safety profile of XEPI in pediatric patients 2 months and older was similar to that of adults [see Adverse Reactions (6.1)].
The safety and effectiveness of XEPI in pediatric patients younger than 2 months of age have not been established [see Clinical Studies (14)].
10. Overdosage
Any sign or symptom of overdose, either topically or by accidental ingestion, should be treated symptomatically. No specific antidote is known.
11. Xepi Cream Description
XEPI contains ozenoxacin, a quinolone antimicrobial. It is intended for topical use only.
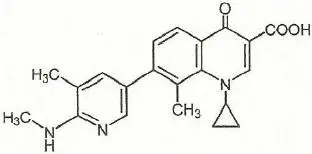
The chemical name of ozenoxacin is 1-Cyclopropyl-8-methyl-7-(5-methyl-6-methylamino-pyridin-3-yl)-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid. Ozenoxacin, a white to pale-yellow crystalline solid, has a molecular formula of C21H21N3O3, and a molecular weight of 363.41. The chemical structure is:
Each gram of cream contains 10 mg of ozenoxacin (1% w/w) and the following inactive ingredients: benzoic acid, octyldodecanol, peglicol 5 oleate, pegoxol 7 stearate, propylene glycol, purified water, stearyl alcohol.
12. Xepi Cream - Clinical Pharmacology
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate carcinogenic potential have not been conducted with ozenoxacin.
Ozenoxacin demonstrated no genotoxicity when evaluated in vitro for gene mutation and/or chromosomal effects in the Ames test, mouse lymphoma cell assay, or when evaluated in vivo in a rat micronucleus test with demonstrated systemic exposure.
Oral doses of ozenoxacin did not affect mating and fertility in male and female rats treated up to 500 mg/kg/day (about 8500 and 16,000 times respectively, the maximum human plasma concentration seen with dermal application of ozenoxacin 1% cream).
14. Clinical Studies
The safety and efficacy of XEPI for the treatment of impetigo was evaluated in two multi-center, randomized, double-blind placebo controlled clinical trials (Trial 1, (NCT01397461) and Trial 2, (NCT02090764)). Seven-hundred twenty-three (723) subjects two months of age and older with an affected body surface area of up to 100 cm2, and not exceeding 2% for subjects aged 2 months to 11 years were randomized to XEPI or placebo. Subjects applied XEPI or placebo twice daily for 5 days. Subjects with underlying skin disease (e.g., preexisting eczematous dermatitis), skin trauma, clinical evidence of secondary infection, or systemic signs and symptoms of infection (such as fever), were excluded from these studies.
Overall clinical success was defined as no need for additional antimicrobial therapy of the baseline affected area(s) and absence/reduction in clinical signs and symptoms assessed at the end of therapy (Day 6-7), as follows: absence of exudates/pus, crusting, tissue warmth, and pain; and erythema/inflammation, tissue edema, and itching assessed as less than mild in Trial 1; and absence of blistering, exudates/pus, crusting, and itching/pain, and mild or improved erythema/inflammation in Trial 2. Table 2 below presents the results for clinical response at the end of therapy.
| Trial 1 | Trial 2 | |||
|---|---|---|---|---|
| XEPI | Placebo | XEPI | Placebo | |
| (N = 155) n (%) | (N = 156) n (%) | (N = 206) n (%) | (N = 206) n (%) |
|
| a The success rates for ozenoxacin were significantly different than placebo in Study 1 and Study 2 (p = 0.002 and p = 0.001). | ||||
| Clinical success | 54 (34.8) | 30 (19.2) | 112 (54.4) | 78 (37.9) |
| Clinical failure | 98 (63.2) | 120 (76.9) | 91 (44.2) | 121 (58.7) |
| Unable to determine | 3 (1.9) | 6 (3.8) | 3 (1.5) | 7 (3.4) |
The most commonly identified bacteria were S. aureus and S. pyogenes. Table 3 below presents the results for clinical success at end of therapy in subjects with S.aureus or S.pyogenes at baseline.
| Trial 1 | Trial 2 | |||
|---|---|---|---|---|
| XEPI | Placebo | XEPI | Placebo | |
| Clinical success | n/N (%) | n/N (%) | n/N (%) | n/N (%) |
| S. aureus | 35/93 (37.6) | 16/98 (16.3) | 66/115 (57.4) | 36/108 (33.3) |
| S. pyogenes | 29/73 (39.7) | 7/67 (10.4) | 15/19 (78.9) | 8/20 (40.0) |
16. How is Xepi Cream supplied
XEPI cream, 1% is a pale yellow cream supplied in a 30-gram tube. Each gram of cream contains 10 mg of ozenoxacin.
NDC 70621-103-01 (30-gram tube)
NDC 70621-103-10 (Cardbox containing one 30-gram tube)
17. Patient Counseling Information
Advise patients (and/or their caregivers or guardians) using XEPI of the following information and instructions:
- Use XEPI as directed by the healthcare practitioner. As with any topical medication, patients and caregivers should wash their hands after application if the hands are not the area for treatment.
- XEPI is for external use only.Do not swallow XEPI or use it in the eyes, on the mouth or lips, inside the nose, or inside the female genital area.
- The treated area may be covered by a sterile bandage or gauze dressing.
- Use the medication for the entire time recommended by the healthcare practitioner, even though symptoms may have improved.
- Notify the healthcare practitioner if there is no improvement in symptoms within 3 days after starting use of XEPI.
| XEPI
ozenoxacin cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Biofrontera Inc. (080213133) |





