Drug Detail:Yosprala (Aspirin and omeprazole [ as-pir-in-and-oh-mep-ra-zole ])
Drug Class: Platelet aggregation inhibitors
Highlights of Prescribing Information
YOSPRALA (aspirin and omeprazole) delayed-release tablets, for oral use
Initial U.S. Approval: 2016
Recent Major Changes
| Warnings and Precautions, Fundic Gland Polyps (5.19) | 06/2018 |
Indications and Usage for Yosprala
YOSPRALA is a combination of aspirin, an anti-platelet agent, and omeprazole, a proton pump inhibitor (PPI), indicated for patients who require aspirin for secondary prevention of cardiovascular and cerebrovascular events and who are at risk of developing aspirin associated gastric ulcers.
The aspirin component of YOSPRALA is indicated for:
- reducing the combined risk of death and nonfatal stroke in patients who have had ischemic stroke or transient ischemia of the brain due to fibrin platelet emboli,
- reducing the combined risk of death and nonfatal MI in patients with a previous MI or unstable angina pectoris,
- reducing the combined risk of MI and sudden death in patients with chronic stable angina pectoris,
- use in patients who have undergone revascularization procedures (Coronary Artery Bypass Graft [CABG] or Percutaneous Transluminal Coronary Angioplasty [PTCA]) when there is a pre-existing condition for which aspirin is already indicated.
The omeprazole component of YOSPRALA is indicated for decreasing the risk of developing aspirin associated gastric ulcers in patients at risk for developing aspirin-associated gastric ulcers due to age (≥ 55) or documented history of gastric ulcers. (1)
Limitations of Use:
- Not for use as the initial dose of aspirin therapy during onset of acute coronary syndrome, acute myocardial infarction or before percutaneous coronary intervention. (1)
- Has not been shown to reduce the risk of gastrointestinal bleeding due to aspirin. (1)
- Do not substitute YOSPRALA with the single-ingredient products of aspirin and omeprazole. (1)
Yosprala Dosage and Administration
- Recommended dosage: One tablet daily at least 60 minutes before a meal. (2.1, 2.2)
- Do not split, chew, crush or dissolve the tablet. (2.2)
Dosage Forms and Strengths
Delayed-Release Tablets (3):
- 81 mg delayed-release aspirin/40 mg immediate-release omeprazole
- 325 mg delayed-release aspirin/40 mg immediate-release omeprazole
Contraindications
- History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs. (4)
- In pediatric patients with suspected viral infections, with or without fever, because of the risk of Reye's Syndrome. (4)
- Known hypersensitivity to aspirin, omeprazole, substituted benzimidazoles or to any of the excipients of YOSPRALA. (4)
- Patients receiving rilpivirine-containing products. (4, 7)
Warnings and Precautions
- Coagulation Abnormalities: Risk of increased bleeding time with aspirin, especially in patients with inherited (hemophilia) or acquired (liver disease or vitamin K deficiency) bleeding disorders. Monitor patients for signs of increased bleeding. (5.1)
- GI Adverse Reactions (including ulceration and bleeding): Monitor for signs and symptoms and discontinue treatment if bleeding occurs. (5.2)
- Bleeding Risk with Use of Alcohol: Avoid heavy alcohol use (three or more drinks every day). (5.3)
- Reduction in Antiplatelet Activity with Clopidogrel due to Interference with CYP2C19 Metabolism: Consider other antiplatelet therapy. (5.4, 7)
- Reduction in Efficacy of Ticagrelor: Avoid use with the 325/40 strength of YOSPRALA. (5.5, 7)
- Renal Failure: Avoid YOSPRALA in patients with severe renal failure. (5.6, 8.6)
- Gastric Malignancy: In adults, response to gastric symptoms does not preclude the presence of gastric malignancy; Consider additional follow-up and diagnostic testing. (5.7)
- Acute Interstitial Nephritis: Observed in patients taking PPIs. (5.8)
- Clostridium difficile-Associated Diarrhea: PPI therapy may be associated with increased risk; use lowest dose and shortest duration of treatment. (5.9)
- Bone Fracture: Long-term and multiple daily dose PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist or spine; use lowest dose and shortest duration of treatment. (5.10)
- Cutaneous and Systemic Lupus Erythematosus: Mostly cutaneous; new onset or exacerbation of existing disease; discontinue YOSPRALA and refer to specialist for evaluation. (5.11)
- Hepatic Impairment: Avoid YOSPRALA in patients with all degrees of hepatic impairment. (5.12, 8.7)
- Cyanocobalamin (Vitamin B-12) Deficiency: Daily long-term use (e.g., longer than 3 years) of PPI may lead to malabsorption or deficiency. (5.13)
- Hypomagnesemia: Reported rarely with prolonged treatment with PPIs; consider monitoring magnesium levels. (5.14)
- Reduced Effect of Omeprazole with St. John's Wort or Rifampin: Avoid concomitant use. (5.15, 7)
- Interactions with Diagnostic Investigations for Neuroendocrine Tumors: Increased Chromogranin A (CgA) levels may interfere with diagnostic investigations for neuroendocrine tumors; temporarily stop YOSPRALA at least 14 days before assessing CgA levels (5.16, 7)
- Bone Marrow Toxicity with Methotrexate, especially in the elderly or renally impaired: Use with PPIs may elevate and/or prolong serum levels of methotrexate and/or its metabolite, possibly leading to toxicity. With high dose methotrexate, consider a temporary withdrawal of YOSPRALA. (5.17, 7)
- Premature closure of the ductus arteriosus: Avoid use in pregnant women starting at 30 weeks gestation. (5.18, 8.1)
- Abnormal Laboratory Tests: Aspirin has been associated with elevated hepatic enzymes, blood urea nitrogen and serum creatinine, hyperkalemia, proteinuria, and prolonged bleeding time. (5.19)
- Fundic Gland Polyps: Risk increases with long-term use, especially beyond one year. Use the shortest duration of therapy. (5.20)
Drug Interactions
See full prescribing information for a list of clinically important drug interactions. (7)
Use In Specific Populations
- Lactation: Breastfeeding not recommended. (8.2)
- Females and Males of Reproductive Potential Infertility: NSAIDs are associated with reversible infertility. Consider withdrawal of YOSPRALA in women who have difficulties conceiving. (8.3)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2020
Full Prescribing Information
1. Indications and Usage for Yosprala
YOSPRALA, a combination of aspirin and omeprazole, is indicated for patients who require aspirin for secondary prevention of cardiovascular and cerebrovascular events and who are at risk of developing aspirin associated gastric ulcers.
The aspirin component of YOSPRALA is indicated for:
- reducing the combined risk of death and nonfatal stroke in patients who have had ischemic stroke or transient ischemia of the brain due to fibrin platelet emboli,
- reducing the combined risk of death and nonfatal MI in patients with a previous MI or unstable angina pectoris,
- reducing the combined risk of MI and sudden death in patients with chronic stable angina pectoris,
- use in patients who have undergone revascularization procedures (Coronary Artery Bypass Graft [CABG] or Percutaneous Transluminal Coronary Angioplasty [PTCA]) when there is a pre-existing condition for which aspirin is already indicated.
The omeprazole component of YOSPRALA is indicated for decreasing the risk of developing aspirin-associated gastric ulcers in patients at risk for developing aspirin-associated gastric ulcers due to age (≥ 55) or documented history of gastric ulcers.
2. Yosprala Dosage and Administration
2.1 Recommended Dosage
- Take one tablet daily.
- YOSPRALA is available in combinations that contain 81 mg or 325 mg of aspirin. Generally 81 mg of aspirin has been accepted as an effective dose for secondary cardiovascular prevention. Providers should consider the need for 325 mg and refer to current clinical practice guidelines.
2.2 Administration Instructions
- Take YOSPRALA once daily at least 60 minutes before a meal.
- The tablets are to be swallowed whole with liquid. Do not split, chew, crush or dissolve the tablet.
- Use the lowest effective dose of YOSPRALA based on the individual patient's treatment goals and to avoid potential dose dependent adverse reactions including bleeding.
- If a dose of YOSPRALA is missed, advise patients to take it as soon as it is remembered. If it is almost time for the next dose, skip the missed dose. Take the next dose at the regular time. Patients should not take 2 doses at the same time unless advised by their doctor.
- Do not stop taking YOSPRALA suddenly as this could increase the risk of heart attack or stroke.
3. Dosage Forms and Strengths
Oval, blue-green, film-coated, delayed-release tablets for oral administration containing either:
- 81 mg delayed-release aspirin and 40 mg immediate-release omeprazole, printed with 81/40, or
- 325 mg delayed-release aspirin and 40 mg immediate-release omeprazole, printed with 325/40.
4. Contraindications
YOSPRALA is contraindicated in:
- Patients with known allergy to aspirin and other nonsteroidal anti-inflammatory drug products (NSAIDs) and in patients with the syndrome of asthma, rhinitis, and nasal polyps. Aspirin may cause severe urticaria, angioedema, or bronchospasm (asthma).
- Pediatric patients with suspected viral infections, with or without fever, because of the risk of Reye's syndrome with concomitant use of aspirin in certain viral illnesses.
- YOSPRALA is contraindicated in patients with known hypersensitivity to aspirin, omeprazole, substituted benzimidazoles, or to any of the excipients in the formulation [see Warnings and Precautions (5.8), Adverse Reactions (6.2)].
- Proton pump inhibitor (PPI)–containing products, including YOSPRALA, are contraindicated in patients receiving rilpivirine-containing products [see Drug Interactions (7)].
5. Warnings and Precautions
5.1 Coagulation Abnormalities
Even low doses of aspirin can inhibit platelet function leading to an increase in bleeding time. This can adversely affect patients with inherited (hemophilia) or acquired (liver disease or vitamin K deficiency) bleeding disorders. Monitor patients for signs of increased bleeding.
5.2 Gastrointestinal Adverse Reactions
Aspirin is associated with serious gastrointestinal (GI) adverse reactions, including inflammation, bleeding ulceration and perforation of the upper and lower GI tract. Other adverse reactions with aspirin include stomach pain, heartburn, nausea, and vomiting.
Serious GI adverse reactions reported in the clinical trials of YOSPRALA were: gastric ulcer hemorrhage in one of the 521 patients treated with YOSPRALA and duodenal ulcer hemorrhage in one of the 524 patients treated with enteric-coated aspirin. In addition, there were two cases of intestinal hemorrhage, one in each treatment group, and one patient treated with YOSPRALA experienced obstruction of the small bowel.
Although minor upper GI symptoms, such as dyspepsia, are common and can occur anytime during therapy, monitor patients for signs of ulceration and bleeding, even in the absence of previous GI symptoms. Inform patients about the signs and symptoms of GI adverse reactions.
If active and clinically significant bleeding from any source occurs in patients receiving YOSPRALA, discontinue treatment.
5.3 Bleeding Risk with Use of Alcohol
Counsel patients who consume three or more alcoholic drinks every day about the bleeding risks involved with chronic, heavy alcohol use while taking YOSPRALA.
5.4 Interaction with Clopidogrel
Avoid concomitant use of YOSPRALA with clopidogrel. Clopidogrel is a prodrug. Inhibition of platelet aggregation by clopidogrel is entirely due to an active metabolite. The metabolism of clopidogrel to its active metabolite can be impaired by use with concomitant medications, such as omeprazole, that interfere with CYP2C19 activity. Co-administration of clopidogrel with 80 mg omeprazole reduces the pharmacological activity of clopidogrel, even when administered 12 hours apart. When using YOSPRALA, consider alternative anti-platelet therapy [see Drug Interactions (7), Clinical Pharmacology (12.3)].
5.5 Interaction with Ticagrelor
Maintenance doses of aspirin above 100 mg reduce the effectiveness of ticagrelor in preventing thrombotic cardiovascular events. Avoid concomitant use of ticagrelor with the 325 mg/40 mg tablet strength of YOSPRALA [see Drug Interactions (7)].
5.6 Renal Failure
Avoid YOSPRALA in patients with severe renal failure (glomerular filtration rate less than 10 mL/minute). Regular use of aspirin is associated in a dose-dependent manner with an increased risk of chronic renal failure. Aspirin use decreases glomerular filtration rate and renal blood flow especially with patients with pre-existing renal disease. [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
5.7 Presence of Gastric Malignancy
In adults, response to gastric symptoms with YOSPRALA does not preclude the presence of gastric malignancy. Consider additional gastrointestinal follow-up and diagnostic testing in adult patients who experience gastric symptoms during treatment with YOSPRALA or have a symptomatic relapse after completing treatment. In older patients, also consider an endoscopy.
5.8 Acute Interstitial Nephritis
Acute interstitial nephritis has been observed in patients taking PPIs including omeprazole. Acute interstitial nephritis may occur at any point during PPI therapy and is generally attributed to an idiopathic hypersensitivity reaction. Discontinue YOSPRALA if acute interstitial nephritis develops [see Contraindications (4)].
5.9 Clostridium difficile-Associated Diarrhea
Published observational studies suggest that PPI-containing therapy like YOSPRALA may be associated with an increased risk of Clostridium difficile-associated diarrhea (CDAD), especially in hospitalized patients. This diagnosis should be considered for diarrhea that does not improve [see Adverse Reactions (6.2)].
Use the lowest dose and shortest duration of YOSPRALA appropriate to the condition being treated.
5.10 Bone Fracture
Several published observational studies suggest that PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist, or spine. The risk of fracture was increased in patients who received high-dose, defined as multiple daily doses, and long-term PPI therapy (a year or longer). Use the lowest dose and shortest duration of YOSPRALA therapy appropriate to the condition being treated. Manage patients at risk for osteoporosis-related fractures according to established treatment guidelines [see Adverse Reactions (6.2)].
5.11 Cutaneous and Systemic Lupus Erythematosus
Cutaneous lupus erythematosus (CLE) and systemic lupus erythematosus (SLE) have been reported in patients taking PPIs, including omeprazole. These events have occurred as both new onset and an exacerbation of existing autoimmune disease. The majority of PPI-induced lupus erythematous cases were CLE.
The most common form of CLE reported in patients treated with PPIs was subacute CLE (SCLE), and occurred within weeks to years after continuous drug therapy in patients ranging from infants to the elderly.Generally, histological findings were observed without organ involvement.
Systemic lupus erythematosus (SLE) is less commonly reported than CLE in patients receiving PPIs. PPI associated SLE is usually milder than non-drug induced SLE. Onset of SLE typically occurred within days to years after initiating treatment, but some cases occurred days or years after initiating treatment. SLE occurred primarily in patients ranging from young adults to the elderly. The majority of patients presented with rash; however, arthralgia and cytopenia were also reported.
Avoid administration of PPIs for longer than medically indicated. If signs or symptoms consistent with CLE or SLE are noted in patients receiving YOSPRALA, discontinue the drug and refer the patient to the appropriate specialist for evaluation. Most patients improve with discontinuation of the PPI alone in 4 to 12 weeks. Serologicial testing (e.g., ANA) may be positive and elevated serologicial test results may take longer to resolve than clinical manifestations.
5.12 Hepatic Impairment
Long-term moderate to high doses of aspirin may result in elevations in serum ALT levels. These abnormalities resolve rapidly with discontinuation of aspirin. The hepatotoxicity of aspirin is usually mild and asymptomatic. Bilirubin elevations are usually mild or absent. Systemic exposure to omeprazole is increased in patients with hepatic impairment [see Clinical Pharmacology (12.3)]. Avoid YOSPRALA in patients with any degree of hepatic impairment [see Use in Specific Populations (8.7)].
5.13 Cyanocobalamin (Vitamin B-12) Deficiency
Daily treatment with any acid-suppressing medications over a long period of time (e.g., longer than 3 years) may lead to malabsorption of cyanocobalamin (vitamin B-12) caused by hypo- or achlorhydria. Rare reports of cyanocobalamin deficiency occurring with acid-suppressing therapy have been reported in the literature. This diagnosis should be considered if clinical symptoms consistent with cyanocobalamin deficiency are observed in patients treated with YOSPRALA.
5.14 Hypomagnesemia
Hypomagnesemia, symptomatic and asymptomatic, has been reported rarely in patients treated with PPIs for at least three months, in most cases after a year of therapy. Serious adverse events include tetany, arrhythmias, and seizures. In most patients, treatment of hypomagnesemia required magnesium replacement and discontinuation of the PPI. For patients expected to be on prolonged treatment or who take YOSPRALA with medications such as digoxin or drugs that may cause hypomagnesemia (e.g., diuretics), consider monitoring magnesium levels prior to initiation of YOSPRALA and periodically during treatment [see Adverse Reactions (6.2)].
5.15 Reduced Effect of Omeprazole with St. John's Wort or Rifampin
Drugs which induce the CYP2C19 or CYP3A4 (such as St. John's Wort or rifampin) can substantially decrease concentrations of omeprazole. Avoid concomitant use of YOSPRALA with St. John's Wort or rifampin [see Drug Interactions (7)].
5.16 Interactions with Diagnostic Investigations for Neuroendocrine Tumors
Serum chromogranin A (CgA) levels increase secondary to omeprazole-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic interventions for neuroendocrine tumors. Temporarily discontinue treatment with YOSPRALA at least 14 days before assessing CgA levels and consider repeating the test if initial CgA levels are high. If serial tests are performed (e.g., for monitoring), the same commercial laboratory should be used for testing, as reference ranges between tests may vary [see Drug Interactions (7) and Clinical Pharmacology (12.2)].
5.17 Interaction with Methotrexate
Literature suggests that concomitant use of PPIs with methotrexate (primarily at high dose) may elevate and prolong serum levels of methotrexate and/or its metabolite, possibly leading to methotrexate toxicities. In high-dose methotrexate administration, a temporary withdrawal of YOSPRALA may be considered in some patients [see Drug Interactions (7)].
5.18 Premature Closure of Fetal Ductus Arteriosus
NSAIDs including aspirin, may cause premature closure of the fetal ductus arteriosus. Avoid use of NSAIDs, including YOSPRALA, in pregnant women starting at 30 weeks of gestation (third trimester). [see Use in Specific Populations (8.1)].
5.19 Abnormal Laboratory Tests
Aspirin has been associated with elevated hepatic enzymes, blood urea nitrogen and serum creatinine, hyperkalemia, proteinuria, and prolonged bleeding time.
5.20 Fundic Gland Polyps
PPI use is associated with an increased risk of fundic gland polyps that increases with long-term use, especially beyond one year. Most PPI users who developed fundic gland polyps were asymptomatic and fundic gland polyps were identified incidentally on endoscopy. Use the shortest duration of PPI therapy appropriate to the condition being treated.
7. Drug Interactions
Tables 2 and 3 include drugs with clinically important drug interactions and interaction with diagnostics when administered concomitantly with YOSPRALA and instructions for preventing or managing them.
Consult the labeling of concomitantly used drugs to obtain further information about interactions with omeprazole or aspirin.
| Antiretrovirals | |
| Clinical Impact: | The effect of PPIs, such as omeprazole, on antiretroviral drugs is variable. The clinical importance and the mechanisms behind these interactions are not always known.
|
| Intervention: | Rilpivirine-containing products: Concomitant use with YOSPRALA is contraindicated [see Contraindications (4)].
Atazanavir: Avoid concomitant use with YOSPRALA. See prescribing information for atazanavir for dosing information. Nelfinavir: Avoid concomitant use with YOSPRALA. See prescribing information for nelfinavir. Saquinavir: See the prescribing information for saquinavir for monitoring of potential saquinavir-related toxicities. Other antiretrovirals: See prescribing information for specific antiretroviral drugs. |
| Heparin and Warfarin | |
| Clinical Impact: | Increased INR and prothrombin time in patients receiving PPIs, including omeprazole, and warfarin concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death. Aspirin can increase the anticoagulant activity of heparin and warfarin, increasing bleeding risk. |
| Intervention: | Monitor INR and prothrombin time and adjust the dose of warfarin, if needed, to maintain target INR range. Monitor and adjust the dose of heparin and warfarin as needed. |
| Methotrexate | |
| Clinical Impact: | Concomitant use of omeprazole with methotrexate (primarily at high dose) may elevate and prolong serum concentrations of methotrexate and/or its metabolite hydroxymethotrexate, possibly leading to methotrexate toxicities. No formal drug interaction studies of high-dose methotrexate with PPIs, such as omeprazole, have been conducted [see Warnings and Precautions (5.17)]. |
| Intervention: | A temporary withdrawal of YOSPRALA may be considered in some patients receiving high-dose methotrexate. |
| CYP2C19 Substrates (e.g., clopidogrel, citalopram, cilostazol, phenytoin, diazepam) | |
| Clopidogrel | |
| Clinical Impact: | Concomitant use of omeprazole 80 mg results in reduced plasma concentrations of the active metabolite of clopidogrel and a reduction in platelet inhibition [see Clinical Pharmacology (12.3)].
There are no adequate combination studies of a lower dose of omeprazole or a higher dose of clopidogrel in comparison with the approved dose of clopidogrel. |
| Intervention: | Avoid concomitant use with YOSPRALA. Consider use of alternative anti-platelet therapy [see Warnings and Precautions (5.4)]. |
| Citalopram | |
| Clinical Impact: | Concomitant use of omeprazole results in increased exposure of citalopram leading to an increased risk of QT prolongation [see Clinical Pharmacology (12.3)]. |
| Intervention: | Limit the dose of citalopram to a maximum of 20 mg per day. See prescribing information for citalopram. |
| Cilostazol | |
| Clinical Impact: | Concomitant use of omeprazole results in increased exposure of one of the active metabolites of cilostazol (3,4-dihydro-cilostazol). |
| Intervention: | Reduce the dose of cilostazol to 50 mg twice daily. See prescribing information for cilostazol. |
| Phenytoin | |
| Clinical Impact: | Potential for increased exposure of phenytoin with concomitant omeprazole. Aspirin can displace protein-bound phenytoin leading to a decrease in the total concentration of phenytoin. |
| Intervention: | Monitor phenytoin serum concentrations. Dose adjustment may be needed to maintain therapeutic drug concentrations. See prescribing information for phenytoin. |
| Diazepam | |
| Clinical Impact: | Increased exposure of diazepam with concomitant omeprazole [see Clinical Pharmacology (12.3)]. |
| Intervention: | Monitor patients for increased sedation and reduce the dose of diazepam as needed. |
| Ticagrelor | |
| Clinical Impact: | Maintenance doses of aspirin above 100 mg reduce the effectiveness of ticagrelor. |
| Intervention: | Avoid concomitant use of ticagrelor with the 325 mg/40 mg tablet strength of YOSPRALA [see Warnings and Precautions (5.5)]. |
| Digoxin | |
| Clinical Impact: | Potential for increased exposure of digoxin with concomitant omeprazole [see Clinical Pharmacology (12.3)]. |
| Intervention: | Monitor digoxin concentrations. Dose adjustment may be needed to maintain therapeutic drug concentrations. See digoxin prescribing information. |
| Drugs Dependent on Gastric pH for Absorption (e.g., iron salts, erlotinib, dasatinib, nilotinib, mycophenolate mofetil, ketoconazole/itraconazole) | |
| Clinical Impact: | Omeprazole can reduce the absorption of other drugs due to its effect on reducing intragastric acidity. |
| Intervention: | Mycophenolate mofetil (MMF): Co-administration of omeprazole in healthy subjects and in transplant patients receiving MMF has been reported to reduce the exposure to the active metabolite, mycophenolic acid (MPA), possibly due to a decrease in MMF solubility at an increased gastric pH. The clinical relevance of reduced MPA exposure on organ rejection has not been established in transplant patients receiving omeprazole and MMF. Use Yosprala with caution in transplant patients receiving MMF [see Clinical Pharmacology (12.3)].
See the prescribing information for other drugs dependent on gastric pH for absorption. |
| Tacrolimus | |
| Clinical Impact: | Potential for increased exposure of tacrolimus with concomitant omeprazole, especially in transplant patients who are intermediate or poor metabolizers of CYP2C19. |
| Intervention: | Monitor tacrolimus whole blood concentrations. Dose adjustment may be needed to maintain therapeutic drug concentrations. See prescribing information for tacrolimus. |
| ACE-Inhibitors | |
| Clinical Impact: | Aspirin may diminish the antihypertensive effect of ACE-inhibitors. |
| Intervention: | Monitor blood pressure as needed. |
| Beta Blockers | |
| Clinical Impact: | The hypotensive effects of beta blockers may be diminished by the concomitant administration of aspirin. |
| Intervention: | Monitor blood pressure as needed in patients taking YOSPRALA concomitantly with beta blockers for hypertension. |
| Diuretics | |
| Clinical Impact: | The effectiveness of diuretics in patients with underlying renal or cardiovascular disease may be diminished by the concomitant administration of aspirin. |
| Intervention: | Monitor blood pressure as needed. |
| NSAIDs | |
| Clinical Impact: | The concurrent use of NSAIDs and aspirin may increase the risk of serious adverse events, including increased bleeding or decreased renal function. |
| Intervention: | Monitor for signs and symptoms of bleeding or decreased renal function. |
| Oral Hypoglycemics | |
| Clinical Impact: | Moderate doses of aspirin may increase the effectiveness of oral hypoglycemic drugs, leading to hypoglycemia. |
| Intervention: | Monitor blood sugar as needed. |
| Acetazolamide | |
| Clinical Impact: | Concurrent use of aspirin and acetazolamide can lead to high serum concentrations of acetazolamide (and toxicity). |
| Intervention: | Adjust the dose of acetazolamide if signs of toxicity occur. |
| Uricosuric Agents (Probenecid) | |
| Clinical Impact: | Aspirin antagonizes the uricosuric action of uricosuric agents. |
| Intervention: | Monitor serum uric acid levels as needed. |
| Valproic Acid | |
| Clinical Impact: | Concomitant use of aspirin can displace protein-bound valproic acid, leading to an increase in serum concentrations of valproic acid. |
| Intervention: | Monitor valproic acid serum concentrations. Dose adjustment may be needed to maintain therapeutic drug concentrations. See prescribing information for valproic acid. |
| Interactions with Investigations of Neuroendocrine Tumors | |
| Clinical Impact: | Serum chromogranin A (CgA) levels increase secondary to omeprazole-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic investigations for neuroendocrine tumors [see Warnings and Precautions (5.16), Clinical Pharmacology (12.2)]. |
| Intervention: | Temporarily stop YOSPRALA treatment at least 14 days before assessing CgA levels and consider repeating the test if initial CgA levels are high. If serial tests are performed (e.g., for monitoring), the same commercial laboratory should be used for testing, as reference ranges between tests may vary. |
| Interaction with Secretin Stimulation Test | |
| Clinical Impact: | Omeprazole can cause a hyper-response in gastrin secretion in response to secretin stimulation test, falsely suggesting gastrinoma. |
| Intervention: | Temporarily stop YOSPRALA treatment at least 14 days before assessing to allow gastrin levels to return to baseline [see Clinical Pharmacology (12.2)]. |
| False Positive Urine Tests for THC | |
| Clinical Impact: | There have been reports of false positive urine screening tests for tetrahydrocannabinol (THC) in patients receiving PPIs such as omeprazole. |
| Intervention: | An alternative confirmatory method should be considered to verify positive results. |
| Other | |
| Clinical Impact: | There have been clinical reports of interactions with other drugs metabolized via the cytochrome P450 system (e.g., cyclosporine, disulfiram) with omeprazole. |
| Intervention: | Monitor patients to determine if it is necessary to adjust the dosage of these other drugs when taken concomitantly with YOSPRALA. |
| CYP2C19 or CYP3A4 Inducers | |
| Clinical Impact: | Decreased exposure of omeprazole when used concomitantly with strong inducers [see Clinical Pharmacology (12.3)]. |
| Intervention: | St. John's Wort, rifampin: Avoid concomitant use with YOSPRALA [see Warnings and Precautions (5.15)].
Ritonavir-containing products: See prescribing information for specific drugs. |
| CYP2C19 or CYP3A4 Inhibitors | |
| Clinical Impact: | Increased exposure of omeprazole [see Clinical Pharmacology (12.3)]. |
| Intervention: | Voriconazole: Avoid concomitant use with YOSPRALA. |
8. Use In Specific Populations
8.1 Pregnancy
8.4 Pediatric Use
The safety and efficacy of YOSPRALA has not been established in pediatric patients. YOSPRALA is contraindicated in pediatric patients with suspected viral infections, with or without fever, because of the risk of Reye's syndrome with concomitant use of aspirin in certain viral illnesses [see Contraindications (4)].
8.5 Geriatric Use
Of the total number of patients who received YOSPRALA (n=900) in clinical trials, 62% were ≥65 years of age and 15% were 75 years and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects and other reported clinical experience with aspirin and omeprazole has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
No dose reduction of YOSPRALA is necessary in patients with mild to moderate renal impairment. Avoid YOSPRALA in patients with severe renal impairment (glomerular filtration rate less than 10 mL/minute) due to the aspirin component [see Warnings and Precautions (5.6), Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Long-term moderate to high doses of aspirin may result in elevations in serum ALT levels [see Warnings and Precautions (5.12)]. Systemic exposure to omeprazole is increased in patients with hepatic impairment [see Clinical Pharmacology (12.3)]. Avoid YOSPRALA in patients with any degree of hepatic impairment.
8.8 Asian Population
In studies of healthy subjects, Asians had approximately a four-fold higher exposure to omeprazole than Caucasians. CYP2C19, a polymorphic enzyme, is involved in the metabolism of omeprazole. Approximately 15% to 20% of Asians are CYP2C19 poor metabolizers. Tests are available to identify a patient's CYP2C19 genotype. Avoid use in Asian patients with unknown CYP2C19 genotype or those who are known to be poor metabolizers [see Clinical Pharmacology (12.5)].
10. Overdosage
There is no clinical data on overdosage with YOSPRALA.
11. Yosprala Description
The active ingredients of YOSPRALA are aspirin which is an antiplatelet agent and omeprazole which is a PPI.
YOSPRALA (aspirin and omeprazole) is an oval, blue-green, multi-layer film-coated, delayed-release tablet consists of an enteric coated delayed-release aspirin core surrounded by an immediate-release omeprazole layer for oral administration. Each delayed-release tablet contains either 81 mg aspirin and 40 mg omeprazole printed with 81/40, or 325 mg aspirin and 40 mg omeprazole printed with 325/40.
The excipients used in the formulation of YOSPRALA are all inactive and United States Pharmacopeia/National Formulary (USP/NF) defined. The inactive ingredients in YOSPRALA include: carnauba wax, colloidal silicon dioxide, corn starch, FD&C Blue #2, glyceryl monostearate, hydroxypropyl methycellulose, methacrylic acid copolymer dispersion, microcrystalline cellulose, polydextrose, polyethylene glycol, polysorbate 80, povidone, pre-gelatinized starch, sodium phosphate dibasic anhydrous, stearic acid, talc, titanium dioxide, triacetin, triethyl citrate, yellow iron oxide.
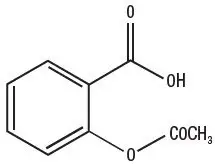
Aspirin is acetylsalicylic acid and is chemically known as benzoic acid, 2-(acetyloxy). Aspirin is an odorless white needle-like crystalline or powdery substance. When exposed to moisture, aspirin hydrolyzes into salicylic and acetic acids and gives off a vinegary odor. It is highly lipid soluble and slightly soluble in water. Aspirin irreversibly inhibits platelet COX-1.
Omeprazole is a white to off-white crystalline powder which melts with decomposition at about 155°C. It is a weak base, freely soluble in ethanol and methanol, and slightly soluble in acetone and isopropanol and very slightly soluble in water. The stability of omeprazole is a function of pH; it is rapidly degraded in acid media, but has acceptable stability under alkaline conditions.
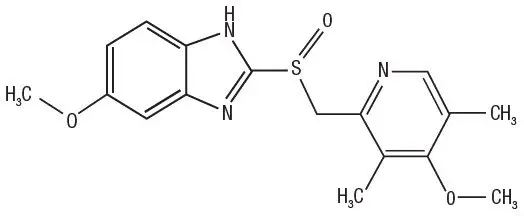
Omeprazole is a substituted benzimidazole, 5-methoxy-2-[[(4-methoxy-3, 5-dimethyl-2-pyridinyl) methyl] sulfinyl]-1H- benzimidazole, a compound that inhibits gastric acid secretion.
12. Yosprala - Clinical Pharmacology
12.1 Mechanism of Action
Aspirin (acetylsalicylic acid) is an inhibitor of both prostaglandin synthesis and platelet aggregation. The differences in activity between aspirin and salicylic acid are thought to be due to the acetyl group on the aspirin molecule. This acetyl group is responsible for the inactivation of cyclo-oxygenase via acetylation.
Omeprazole belongs to a class of antisecretory compounds, the substituted benzimidazoles, that suppress gastric acid secretion by specific inhibition of the [H+/K+]-ATPase enzyme system at the secretory surface of the gastric parietal cell. Because this enzyme system is regarded as the acid (proton) pump within the gastric mucosa, omeprazole has been characterized as a gastric acid-pump inhibitor, in that it blocks the final step of acid production. This effect is dose-related and leads to inhibition of both basal and stimulated acid secretion irrespective of the stimulus.
12.3 Pharmacokinetics
Specific Populations
Drug Interaction Studies
12.5 Pharmacogenomics
CYP2C19, a polymorphic enzyme, is involved in the metabolism of omeprazole. The CYP2C19*1 allele is fully functional while the CYP2C19*2 and *3 alleles are nonfunctional. There are other alleles associated with no or reduced enzymatic function. Patients carrying two fully functional alleles are extensive metabolizers and those carrying two loss-of-function alleles are poor metabolizers. In extensive metabolizers, omeprazole is primarily metabolized by CYP2C19. The systemic exposure to omeprazole varies with a patient's metabolism status: poor metabolizers > intermediate metabolizers > extensive metabolizers. Approximately 3% of Caucasians and 15 to 20% of Asians are CYP2C19 poor metabolizers.
In a pharmacokinetic study of single 20 mg omeprazole dose, the AUC of omeprazole in Asian subjects was approximately four-fold of that in Caucasians [see Use in Specific Populations (8.8)].
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies to evaluate the potential effects of YOSPRALA on carcinogenicity, mutagenicity, or impairment of fertility have not been conducted.
16. How is Yosprala supplied
YOSPRALA (aspirin 81 mg/omeprazole 40 mg) and (aspirin 325 mg/omeprazole 40 mg) delayed-release tablets are oval, blue-green, film-coated tablets printed with 81/40 and 325/40 respectively in black ink. YOSPRALA tablets are packaged in high density polyethylene (HDPE) bottles with desiccants and are supplied as:
| NDC 73568-004-30 | Bottles of 30 tablets | YOSPRALA 81/40 |
| NDC 73568-005-02 | Bottles of 30 tablets | YOSPRALA 325/40 |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Inform patients, families, or caregivers of the following before initiating therapy with YOSPRALA and periodically during the course of ongoing therapy.
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: September 2018 r1 |
|
MEDICATION GUIDE |
|
|
What is the most important information I should know about YOSPRALA? You should take YOSPRALA exactly as prescribed, at the lowest dose possible and for the shortest time needed. YOSPRALA may help reduce the risk of stomach ulcers from aspirin use, but you could still have bleeding and stomach or intestine ulcers, or other serious stomach or intestine problems. Talk with your doctor. Tell your doctor if you have unexpected bleeding, if you bleed more than usual, or if your bleeding lasts longer than is normal for you, such as increased bruising or more frequent nose bleeds. YOSPRALA contains aspirin, a nonsteroidal anti-inflammatory drug (NSAID) and omeprazole, a proton pump inhibitor (PPI) medicine. Before taking YOSPRALA, tell your doctor if you take:
Do not stop taking YOSPRALA without talking with your doctor. Stopping YOSPRALA suddenly could increase your risk of having a heart attack or stroke. YOSPRALA can cause serious side effects, including:
Talk to your doctor about your risk of these serious side effects. YOSPRALA can have other serious side effects. See "What are the possible side effects of YOSPRALA?" |
|
|
What is YOSPRALA? YOSPRALA is a prescription medicine used:
The aspirin in YOSPRALA is used:
The omeprazole in YOSPRALA is used:
YOSPRALA should not be used to treat sudden signs and symptoms of a heart attack or stroke. YOSPRALA should only be used as directed by your doctor to help reduce the risk of further heart problems or strokes. It is not known if YOSPRALA is safe and effective in children. YOSPRALA has not been shown to reduce the risk of bleeding in the stomach or intestines that is caused by aspirin. You should not take an aspirin tablet and an omeprazole tablet together instead of taking YOSPRALA, because they will not work the same way. |
|
|
Do not take YOSPRALA if you:
Do not give YOSPRALA to a child who has a suspected viral infection, even if they do not have a fever. There is a risk of Reye's syndrome with YOSPRALA because it contains aspirin. |
|
|
Before taking YOSPRALA, tell your doctor about all of your medical conditions, including if you: See "What is the most important information I should know about YOSPRALA?"
Tell your doctor about all of the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. YOSPRALA and some other medicines can interact with each other and cause serious side effects. Do not start taking any new medicine without talking to your doctor first. Especially tell your doctor if you take:
|
|
|
How should I take YOSPRALA?
|
|
|
What should I avoid while taking YOSPRALA? Avoid heavy alcohol use during treatment with YOSPRALA. People who drink three or more drinks that contain alcohol every day have a higher risk of bleeding during treatment with YOSPRALA because it contains aspirin. |
|
|
What are the possible side effects of YOSPRALA? YOSPRALA can cause serious side effects, including: See "What is the most important information I should know about YOSPRALA?"
The most common side effects of YOSPRALA include: indigestion or heartburn and stomach-area pain, nausea, diarrhea, and chest pain behind the breastbone, for example, with eating. These are not all the possible side effects of YOSPRALA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
|
How should I store YOSPRALA?
Keep YOSPRALA and all medicines out of the reach of children. |
|
|
General information about the safe and effective use of YOSPRALA Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use YOSPRALA for a condition for which it was not prescribed. Do not give YOSPRALA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your doctor or pharmacist for information about YOSPRALA that is written for health professionals. |
|
|
What are the ingredients in YOSPRALA? Active ingredients: aspirin and omeprazole Inactive ingredients: carnauba wax, colloidal silicon dioxide, corn starch, FD&C Blue #2, glyceryl monostearate, hydroxypropyl methycellulose, methacrylic acid copolymer dispersion, microcrystalline cellulose, polydextrose, polyethylene glycol, polysorbate 80, povidone, pre-gelatinized starch, sodium phosphate dibasic anhydrous, stearic acid, talc, titanium dioxide, triacetin, triethyl citrate, yellow iron oxide. Manufactured for: PHARMACEUTICA LTD.4050 E Cotton Center Blvd Ste 60 Phoenix, Arizona 85040 |
|
70037927
| YOSPRALA
aspirin and omeprazole tablet, film coated |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| YOSPRALA
aspirin and omeprazole tablet, film coated |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - PHARMACEUTIKA LTD (117373005) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon Pharmaceucticals | 005286822 | MANUFACTURE(73568-004, 73568-005) , PACK(73568-004, 73568-005) , LABEL(73568-004, 73568-005) | |