Drug Detail:Zometa (Zoledronic acid [ zoe-le-dron-ik-as-id ])
Drug Class: Bisphosphonates
Highlights of Prescribing Information
ZOMETA® (zoledronic acid) injection, for intravenous use
Initial U.S. Approval: 2001
Recent Major Changes
|
Warnings and Precautions, Embryo-Fetal Toxicity (5.10) |
12/2018 |
Indications and Usage for Zometa
Zometa is a bisphosphonate indicated for the treatment of:
- Hypercalcemia of Malignancy (1.1)
- Patients with multiple myeloma and patients with documented bone metastases from solid tumors, in conjunction with standard antineoplastic therapy. Prostate cancer should have progressed after treatment with at least one hormonal therapy. (1.2)
Limitations of Use: The safety and efficacy of Zometa has not been established for use in hyperparathyroidism or non-tumor-related hypercalcemia.
Zometa Dosage and Administration
Hypercalcemia of Malignancy (2.1)
- 4 mg as a single-use intravenous infusion over no less than 15 minutes
- 4 mg as retreatment after a minimum of 7 days
Multiple myeloma and Bone Metastasis of Solid Tumors (2.2)
- 4 mg as a single-use intravenous infusion over no less than 15 minutes every 3-4 weeks for patients with creatinine clearance of greater than 60 mL/min.
- Reduce the dose for patients with renal impairment.
- Coadminister oral calcium supplements of 500 mg and a multiple vitamin containing 400 international units of vitamin D daily.
Administer through a separate vented infusion line and do not allow to come in contact with any calcium or divalent cation-containing solutions. (2.3)
Dosage Forms and Strengths
Injection: 4 mg/100 mL (0.04 mg/mL) single-dose ready-to-use bottle (3)
Injection: 4 mg/5 mL (0.8 mg/mL) single-dose vial for dilution prior to intravenous infusion (3)
Contraindications
Hypersensitivity to any component of Zometa (4)
Warnings and Precautions
- Patients being treated with Zometa should not be treated with Reclast®. (5.1)
- Adequately rehydrate patients with hypercalcemia of malignancy prior to administration of Zometa and monitor electrolytes during treatment. (5.2)
- Renal toxicity may be greater in patients with renal impairment. Do not use doses greater than 4 mg. Treatment in patients with severe renal impairment is not recommended. Monitor serum creatinine before each dose. (5.3)
- Osteonecrosis of the jaw (ONJ) has been reported. Preventive dental exams should be performed before starting Zometa. Avoid invasive dental procedures. (5.4)
- Severe incapacitating bone, joint, and/or muscle pain may occur. Discontinue Zometa if severe symptoms occur. (5.5)
- Atypical subtrochanteric and diaphyseal femoral fractures have been reported in patients receiving bisphosphonate therapy. These fractures may occur after minimal or no trauma. Evaluate patients with thigh or groin pain to rule out a femoral fracture. Consider drug discontinuation in patients suspected to have an atypical femur fracture. (5.6)
- Hypocalcemia: Correct before initiating Zometa. Adequately supplement patients with calcium and vitamin D. Monitor serum calcium closely with concomitant administration of other drugs known to cause hypocalcemia to avoid severe or life-threatening hypocalcemia. (5.9)
- Zometa can cause fetal harm. Advise females of reproductive potential of potential risk to a fetus and to use effective contraception. (5.10, 8.1, 8.3)
Adverse Reactions/Side Effects
The most common adverse events (greater than 25%) were nausea, fatigue, anemia, bone pain, constipation, fever, vomiting, and dyspnea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Aminoglycosides: May have an additive effect to lower serum calcium for prolonged periods (7.1)
- Loop Diuretics: Concomitant use with Zometa may increase risk of hypocalcemia (7.2)
- Nephrotoxic Drugs: Use with caution (7.3)
Use In Specific Populations
- Lactation: Advise not to breastfeed (8.2)
- Females and Males of Reproductive Potential: Verify pregnancy status prior to initiation of Zometa. May impair fertility. Counsel patients on pregnancy planning and prevention (8.3)
- Pediatric Use: Not indicated for use in pediatric patients (8.4)
- Geriatric Use: Special care to monitor renal function (8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2018
Full Prescribing Information
1. Indications and Usage for Zometa
1.1 Hypercalcemia of Malignancy
Zometa is indicated for the treatment of hypercalcemia of malignancy defined as an albumin-corrected calcium (cCa) of greater than or equal to 12 mg/dL [3.0 mmol/L] using the formula: cCa in mg/dL = Ca in mg/dL + 0.8 (4.0 g/dL - patient albumin [g/dL]).
1.2 Multiple Myeloma and Bone Metastases of Solid Tumors
Zometa is indicated for the treatment of patients with multiple myeloma and patients with documented bone metastases from solid tumors, in conjunction with standard antineoplastic therapy. Prostate cancer should have progressed after treatment with at least one hormonal therapy.
Limitations of Use
The safety and efficacy of Zometa in the treatment of hypercalcemia associated with hyperparathyroidism or with other non–tumor-related conditions have not been established.
2. Zometa Dosage and Administration
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
2.1 Hypercalcemia of Malignancy
The maximum recommended dose of Zometa in hypercalcemia of malignancy (albumin-corrected serum calcium greater than or equal to 12 mg/dL [3.0 mmol/L]) is 4 mg. The 4 mg dose must be given as a single-dose intravenous infusion over no less than 15 minutes. Patients who receive Zometa should have serum creatinine assessed prior to each treatment.
Dose adjustments of Zometa are not necessary in treating patients for hypercalcemia of malignancy presenting with mild-to-moderate renal impairment prior to initiation of therapy (serum creatinine less than 400 µmol/L or less than 4.5 mg/dL).
Patients should be adequately rehydrated prior to administration of Zometa [see Warnings and Precautions (5.2)].
Consideration should be given to the severity of, as well as the symptoms of, tumor-induced hypercalcemia when considering use of Zometa. Vigorous saline hydration, an integral part of hypercalcemia therapy, should be initiated promptly and an attempt should be made to restore the urine output to about 2 L/day throughout treatment. Mild or asymptomatic hypercalcemia may be treated with conservative measures (i.e., saline hydration, with or without loop diuretics). Patients should be hydrated adequately throughout the treatment, but overhydration, especially in those patients who have cardiac failure, must be avoided. Diuretic therapy should not be employed prior to correction of hypovolemia.
Retreatment with Zometa 4 mg may be considered if serum calcium does not return to normal or remain normal after initial treatment. It is recommended that a minimum of 7 days elapse before retreatment, to allow for full response to the initial dose. Renal function must be carefully monitored in all patients receiving Zometa and serum creatinine must be assessed prior to retreatment with Zometa [see Warnings and Precautions (5.2)].
2.2 Multiple Myeloma and Bone Metastases of Solid Tumors
The recommended dose of Zometa in patients with multiple myeloma and metastatic bone lesions from solid tumors for patients with creatinine clearance (CrCl) greater than 60 mL/min is 4 mg infused over no less than 15 minutes every 3 to 4 weeks. The optimal duration of therapy is not known.
Upon treatment initiation, the recommended Zometa doses for patients with reduced renal function (mild and moderate renal impairment) are listed in Table 1. These doses are calculated to achieve the same area under the curve (AUC) as that achieved in patients with creatinine clearance of 75 mL/min. Creatinine clearance is calculated using the Cockcroft-Gault formula [see Warnings and Precautions (5.2)].
| *Doses calculated assuming target AUC of 0.66 (mg•hr/L) (CrCl = 75 mL/min). | |
| Baseline Creatinine Clearance (mL/min) | Zometa Recommended Dose (mg)* |
| greater than 60 | 4 |
| 50-60 | 3.5 |
| 40-49 | 3.3 |
| 30-39 | 3 |
During treatment, serum creatinine should be measured before each Zometa dose and treatment should be withheld for renal deterioration. In the clinical studies, renal deterioration was defined as follows:
For patients with normal baseline creatinine, increase of 0.5 mg/dL
For patients with abnormal baseline creatinine, increase of 1.0 mg/dL
In the clinical studies, Zometa treatment was resumed only when the creatinine returned to within 10% of the baseline value. Zometa should be reinitiated at the same dose as that prior to treatment interruption.
Patients should also be administered an oral calcium supplement of 500 mg and a multiple vitamin containing 400 international units of vitamin D daily.
2.3 Preparation of Solution
Zometa must not be mixed with calcium or other divalent cation-containing infusion solutions, such as Lactated Ringer’s solution, and should be administered as a single intravenous solution in a line separate from all other drugs.
4 mg/100 mL Single-Dose Ready-to-use Bottle
Bottles of Zometa ready-to-use solution for infusion contain overfill allowing for the administration of 100 mL of solution (equivalent to 4 mg zoledronic acid). This solution is ready-to-use and may be administered directly to the patient without further preparation. For single-use only.
To prepare reduced doses for patients with baseline CrCl less than or equal to 60 mL/min, withdraw the specified volume of the Zometa solution from the bottle (see Table 2) and replace with an equal volume of sterile 0.9% Sodium Chloride, USP, or 5% Dextrose Injection, USP. Administer the newly-prepared dose-adjusted solution to the patient by infusion. Follow proper aseptic technique. Properly discard previously withdrawn volume of ready-to-use solution - do not store or reuse.
| Remove and Discard the Following Zometa Ready-to-use Solution (mL) | Replace With the Following Volume of Sterile 0.9% Sodium Chloride, USP or 5% Dextrose Injection, USP (mL) | Dose (mg) |
| 12.0 | 12.0 | 3.5 |
| 18.0 | 18.0 | 3.3 |
| 25.0 | 25.0 | 3.0 |
If not used immediately after dilution with infusion media, for microbiological integrity, the solution should be refrigerated at 2°C to 8°C (36°F–46°F). The refrigerated solution should then be equilibrated to room temperature prior to administration. The total time between dilution, storage in the refrigerator, and end of administration must not exceed 24 hours.
4 mg/5 mL Single-Dose Vial for Dilution Prior to Intravenous Infusion
Zometa 4 mg/5 mL vial for dilution prior to intravenous infusion contains an overfill to allow withdrawal of 5 mL (equivalent to 4 mg zoledronic acid). Zometa (4 mg/5 mL) should immediately be diluted in 100 mL of sterile 0.9% Sodium Chloride, USP, or 5% Dextrose Injection, USP, following proper aseptic technique, and administered to the patient by intravenous infusion. Do not store undiluted Zometa (4 mg/5 mL) in a syringe, to avoid inadvertent injection.
To prepare reduced doses for patients with baseline CrCl less than or equal to 60 mL/min, withdraw the specified volume of the Zometa (4 mg/5 mL) from the vial for the dose required (see Table 3).
| Remove and use Zometa
Volume (mL) | Dose (mg) |
| 4.4 | 3.5 |
| 4.1 | 3.3 |
| 3.8 | 3.0 |
The withdrawn Zometa (4mg/5 mL) solution must be diluted in 100 mL of sterile 0.9% Sodium Chloride, USP, or 5% Dextrose Injection, USP.
If not used immediately after dilution with infusion media, for microbiological integrity, the solution should be refrigerated at 2°C to 8°C (36°F-46°F). The refrigerated solution should then be equilibrated to room temperature prior to administration. The total time between dilution, storage in the refrigerator, and end of administration must not exceed 24 hours.
2.4 Method of Administration
Due to the risk of clinically significant deterioration in renal function, which may progress to renal failure, single doses of Zometa should not exceed 4 mg and the duration of infusion should be no less than 15 minutes [see Warnings and Precautions (5.3)]. In the trials and in postmarketing experience, renal deterioration, progression to renal failure and dialysis, have occurred in patients, including those treated with the approved dose of 4 mg infused over 15 minutes. There have been instances of this occurring after the initial Zometa dose.
3. Dosage Forms and Strengths
Injection: 4 mg/100 mL (0.04 mg/mL) single-dose ready-to-use bottle.
Injection: 4 mg/5 mL (0.8 mg/mL) single-dose vial for dilution prior to intravenous infusion.
4. Contraindications
Hypersensitivity to Zoledronic Acid or Any Components of Zometa
Hypersensitivity reactions including rare cases of urticaria and angioedema, and very rare cases of anaphylactic reaction/shock have been reported [see Adverse Reactions (6.2)].
5. Warnings and Precautions
5.1 Drugs With Same Active Ingredient or in the Same Drug Class
Zometa contains the same active ingredient as found in Reclast® (zoledronic acid). Patients being treated with Zometa should not be treated with Reclast or other bisphosphonates.
5.2 Hydration and Electrolyte Monitoring
Patients with hypercalcemia of malignancy must be adequately rehydrated prior to administration of Zometa. Loop diuretics should not be used until the patient is adequately rehydrated and should be used with caution in combination with Zometa in order to avoid hypocalcemia. Zometa should be used with caution with other nephrotoxic drugs.
Standard hypercalcemia-related metabolic parameters, such as serum levels of calcium, phosphate, and magnesium, as well as serum creatinine, should be carefully monitored following initiation of therapy with Zometa. If hypocalcemia, hypophosphatemia, or hypomagnesemia occur, short-term supplemental therapy may be necessary.
5.3 Renal Impairment
Zometa is excreted intact primarily via the kidney, and the risk of adverse reactions, in particular renal adverse reactions, may be greater in patients with impaired renal function. Safety and pharmacokinetic data are limited in patients with severe renal impairment and the risk of renal deterioration is increased [see Adverse Reactions (6.1)]. Preexisting renal insufficiency and multiple cycles of Zometa and other bisphosphonates are risk factors for subsequent renal deterioration with Zometa. Factors predisposing to renal deterioration, such as dehydration or the use of other nephrotoxic drugs, should be identified and managed, if possible.
Zometa treatment in patients with hypercalcemia of malignancy with severe renal impairment should be considered only after evaluating the risks and benefits of treatment [see Dosage and Administration (2.1)]. In the clinical studies, patients with serum creatinine greater than 400 µmol/L or greater than 4.5 mg/dL were excluded.
Zometa treatment is not recommended in patients with bone metastases with severe renal impairment. In the clinical studies, patients with serum creatinine greater than 265 µmol/L or greater than 3.0 mg/dL were excluded and there were only 8 of 564 patients treated with Zometa 4 mg by 15-minute infusion with a baseline creatinine greater than 2 mg/dL. Limited pharmacokinetic data exists in patients with creatinine clearance less than 30 mL/min [see Clinical Pharmacology (12.3)].
5.4 Osteonecrosis of the Jaw
Osteonecrosis of the jaw (ONJ) has been reported predominantly in cancer patients treated with intravenous bisphosphonates, including Zometa. Many of these patients were also receiving chemotherapy and corticosteroids which may be risk factors for ONJ. The risk of ONJ may increase with duration of exposure to bisphosphonates.
Postmarketing experience and the literature suggest a greater frequency of reports of ONJ based on tumor type (advanced breast cancer, multiple myeloma), and dental status (dental extraction, periodontal disease, local trauma including poorly fitting dentures). Many reports of ONJ involved patients with signs of local infection including osteomyelitis.
Cancer patients should maintain good oral hygiene and should have a dental examination with preventive dentistry prior to treatment with bisphosphonates.
While on treatment, these patients should avoid invasive dental procedures if possible. For patients who develop ONJ while on bisphosphonate therapy, dental surgery may exacerbate the condition. For patients requiring dental procedures, there are no data available to suggest whether discontinuation of bisphosphonate treatment reduces the risk of ONJ. Clinical judgment of the treating physician should guide the management plan of each patient based on individual benefit/risk assessment [see Adverse Reactions (6.2)].
5.5 Musculoskeletal Pain
In postmarketing experience, severe and occasionally incapacitating bone, joint, and/or muscle pain has been reported in patients taking bisphosphonates, including Zometa. The time to onset of symptoms varied from one day to several months after starting the drug. Discontinue use if severe symptoms develop. Most patients had relief of symptoms after stopping. A subset had recurrence of symptoms when rechallenged with the same drug or another bisphosphonate [see Adverse Reactions (6.2)].
5.6 Atypical Subtrochanteric and Diaphyseal Femoral Fractures
Atypical subtrochanteric and diaphyseal femoral fractures have been reported in patients receiving bisphosphonate therapy, including Zometa. These fractures can occur anywhere in the femoral shaft from just below the lesser trochanter to just above the supracondylar flare and are transverse or short oblique in orientation without evidence of comminution. These fractures occur after minimal or no trauma. Patients may experience thigh or groin pain weeks to months before presenting with a completed femoral fracture. Fractures are often bilateral; therefore the contralateral femur should be examined in bisphosphonate-treated patients who have sustained a femoral shaft fracture. Poor healing of these fractures has also been reported. A number of case reports noted that patients were also receiving treatment with glucocorticoids (such as prednisone or dexamethasone) at the time of fracture. Causality with bisphosphonate therapy has not been established.
Any patient with a history of bisphosphonate exposure who presents with thigh or groin pain in the absence of trauma should be suspected of having an atypical fracture and should be evaluated. Discontinuation of Zometa therapy in patients suspected to have an atypical femur fracture should be considered pending evaluation of the patient, based on an individual benefit risk assessment. It is unknown whether the risk of atypical femur fracture continues after stopping therapy.
5.7 Patients With Asthma
While not observed in clinical trials with Zometa, there have been reports of bronchoconstriction in aspirin-sensitive patients receiving bisphosphonates.
5.8 Hepatic Impairment
Only limited clinical data are available for use of Zometa to treat hypercalcemia of malignancy in patients with hepatic insufficiency, and these data are not adequate to provide guidance on dosage selection or how to safely use Zometa in these patients.
5.9 Hypocalcemia
Hypocalcemia has been reported in patients treated with Zometa. Cardiac arrhythmias and neurologic adverse events (seizures, tetany, and numbness) have been reported secondary to cases of severe hypocalcemia. In some instances, hypocalcemia may be life-threatening. Caution is advised when Zometa is administered with drugs known to cause hypocalcemia, as severe hypocalcemia may develop, [see Drug Interactions (7.2)]. Serum calcium should be measured and hypocalcemia must be corrected before initiating Zometa. Adequately supplement patients with calcium and vitamin D.
5.10 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, Zometa can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of zoledronic acid to pregnant rats during organogenesis resulted in fetal malformations and embryo-fetal lethality at maternal exposures that were greater than or equal to 2.4 times the human clinical exposure based on area under the curve (AUC). Bisphosphonates, such as Zometa, are incorporated into the bone matrix, from where they are gradually released over periods of weeks to years. There may be a risk of fetal harm (e.g., skeletal and other abnormalities) if a woman becomes pregnant after completing a course of bisphosphonate therapy. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during and after Zometa treatment [see Use in Specific Populations (8.1, 8.3), Clinical Pharmacology (12.1)].
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Hypercalcemia of Malignancy
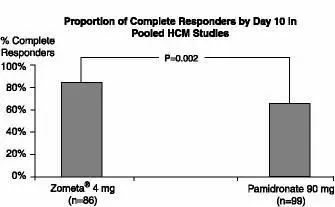
The safety of Zometa was studied in 185 patients with hypercalcemia of malignancy (HCM) who received either Zometa 4 mg given as a 5-minute intravenous infusion (n = 86) or pamidronate 90 mg given as a 2-hour intravenous infusion (n = 103). The population was aged 33-84 years, 60% male and 81% Caucasian, with breast, lung, head and neck, and renal cancer as the most common forms of malignancy. NOTE: pamidronate 90 mg was given as a 2-hour intravenous infusion. The relative safety of pamidronate 90 mg given as a 2-hour intravenous infusion compared to the same dose given as a 24-hour intravenous infusion has not been adequately studied in controlled clinical trials.
Renal Toxicity
Administration of Zometa 4 mg given as a 5-minute intravenous infusion has been shown to result in an increased risk of renal toxicity, as measured by increases in serum creatinine, which can progress to renal failure. The incidence of renal toxicity and renal failure has been shown to be reduced when Zometa 4 mg is given as a 15-minute intravenous infusion. Zometa should be administered by intravenous infusion over no less than 15 minutes [see Warnings and Precautions (5.3), Dosage and Administration (2.4)].
The most frequently observed adverse events were fever, nausea, constipation, anemia, and dyspnea (see Table 4).
Table 4 provides adverse events that were reported by 10% or more of the 189 patients treated with Zometa 4 mg or pamidronate 90 mg from the two HCM trials. Adverse events are listed regardless of presumed causality to study drug.
| Zometa | Pamidronate | |||
| 4 mg | 90 mg | |||
| n (%) | n (%) | |||
| Patients Studied | ||||
| Total No. of Patients Studied | 86 | (100) | 103 | (100) |
| Total No. of Patients with any AE | 81 | (94) | 95 | (92) |
| Body as a Whole | ||||
| Fever | 38 | (44) | 34 | (33) |
| Progression of Cancer | 14 | (16) | 21 | (20) |
| Cardiovascular | ||||
| Hypotension | 9 | (11) | 2 | (2) |
| Digestive | ||||
| Nausea | 25 | (29) | 28 | (27) |
| Constipation | 23 | (27) | 13 | (13) |
| Diarrhea | 15 | (17) | 17 | (17) |
| Abdominal Pain | 14 | (16) | 13 | (13) |
| Vomiting | 12 | (14) | 17 | (17) |
| Anorexia | 8 | (9) | 14 | (14) |
| Hemic and Lymphatic System | ||||
| Anemia | 19 | (22) | 18 | (18) |
| Infections | ||||
| Moniliasis | 10 | (12) | 4 | (4) |
| Laboratory Abnormalities | ||||
| Hypophosphatemia | 11 | (13) | 2 | (2) |
| Hypokalemia | 10 | (12) | 16 | (16) |
| Hypomagnesemia | 9 | (11) | 5 | (5) |
| Musculoskeletal | ||||
| Skeletal Pain | 10 | (12) | 10 | (10) |
| Nervous | ||||
| Insomnia | 13 | (15) | 10 | (10) |
| Anxiety | 12 | (14) | 8 | (8) |
| Confusion | 11 | (13) | 13 | (13) |
| Agitation | 11 | (13) | 8 | (8) |
| Respiratory | ||||
| Dyspnea | 19 | (22) | 20 | (19) |
| Coughing | 10 | (12) | 12 | (12) |
| Urogenital | ||||
| Urinary Tract Infection | 12 | (14) | 15 | (15) |
The following adverse events from the two controlled multicenter HCM trials (n = 189) were reported by a greater percentage of patients treated with Zometa 4 mg than with pamidronate 90 mg and occurred with a frequency of greater than or equal to 5% but less than 10%. Adverse events are listed regardless of presumed causality to study drug: asthenia, chest pain, leg edema, mucositis, dysphagia, granulocytopenia, thrombocytopenia, pancytopenia, nonspecific infection, hypocalcemia, dehydration, arthralgias, headache and somnolence.
Rare cases of rash, pruritus, and chest pain have been reported following treatment with Zometa.
Acute Phase Reaction
Within three days after Zometa administration, an acute phase reaction has been reported in patients, with symptoms including pyrexia, fatigue, bone pain and/or arthralgias, myalgias, chills, and influenza-like illness. These symptoms usually resolve within a few days. Pyrexia has been the most commonly associated symptom, occurring in 44% of patients.
Mineral and Electrolyte Abnormalities
Electrolyte abnormalities, most commonly hypocalcemia, hypophosphatemia, and hypomagnesemia, can occur with bisphosphonate use.
Grade 3 and Grade 4 laboratory abnormalities for serum creatinine, serum calcium, serum phosphorus, and serum magnesium observed in two clinical trials of Zometa in patients with HCM are shown in Table 5 and 6.
| 1Grade 3 (greater than 3x Upper Limit of Normal); Grade 4 (greater than 6x Upper Limit of Normal). 2Grade 3 (less than 7 mg/dL); Grade 4 (less than 6 mg/dL). 3Grade 3 (less than 2 mg/dL); Grade 4 (less than 1 mg/dL). 4Grade 3 (less than 0.8 mEq/L); Grade 4 (less than 0.5 mEq/L). |
||||
| Grade 3 | ||||
| Laboratory Parameter | Zometa | Pamidronate | ||
| 4 mg | 90 mg | |||
| n/N | (%) | n/N | (%) | |
| Serum Creatinine1 | 2/86 | (2%) | 3/100 | (3%) |
| Hypocalcemia2 | 1/86 | (1%) | 2/100 | (2%) |
| Hypophosphatemia3 | 36/70 | (51%) | 27/81 | (33%) |
| Hypomagnesemia4 | 0/71 | 0 | 0/84 | 0 |
| 1Grade 3 (greater than 3x Upper Limit of Normal); Grade 4 (greater than 6x Upper Limit of Normal). 2Grade 3 (less than 7 mg/dL); Grade 4 (less than 6 mg/dL). 3Grade 3 (less than 2 mg/dL); Grade 4 (less than 1 mg/dL). 4Grade 3 (less than 0.8 mEq/L); Grade 4 (less than 0.5 mEq/L). |
||||
| Grade 4 | ||||
| Laboratory Parameter | Zometa | Pamidronate | ||
| 4 mg | 90 mg | |||
| n/N | (%) | n/N | (%) | |
| Serum Creatinine1 | 0/86 | 0 | 1/100 | (1%) |
| Hypocalcemia2 | 0/86 | 0 | 0/100 | 0 |
| Hypophosphatemia3 | 1/70 | (1%) | 4/81 | (5%) |
| Hypomagnesemia4 | 0/71 | 0 | 1/84 | (1%) |
Injection-Site Reactions
Local reactions at the infusion-site, such as redness or swelling, were observed infrequently. In most cases, no specific treatment is required and the symptoms subside after 24-48 hours.
Ocular Adverse Events
Ocular inflammation such as uveitis and scleritis can occur with bisphosphonate use, including Zometa. No cases of iritis, scleritis, or uveitis were reported during these clinical trials. However, cases have been seen in postmarketing use [see Adverse Reactions (6.2)].
Multiple Myeloma and Bone Metastases of Solid Tumors
The safety analysis includes patients treated in the core and extension phases of the trials. The analysis includes the 2042 patients treated with Zometa 4 mg, pamidronate 90 mg, or placebo in the three controlled multicenter bone metastases trials, including 969 patients completing the efficacy phase of the trial, and 619 patients that continued in the safety extension phase. Only 347 patients completed the extension phases and were followed for 2 years (or 21 months for the other solid tumor patients). The median duration of exposure for safety analysis for Zometa 4 mg (core plus extension phases) was 12.8 months for breast cancer and multiple myeloma, 10.8 months for prostate cancer, and 4.0 months for other solid tumors.
Table 7 describes adverse events that were reported by 10% or more of patients. Adverse events are listed regardless of presumed causality to study drug.
| Zometa | Pamidronate | Placebo | ||||
| 4 mg | 90 mg | |||||
| n (%) | n (%) | n (%) | ||||
| Patients Studied | ||||||
| Total No. of Patients | 1031 | (100) | 556 | (100) | 455 | (100) |
| Total No. of Patients with any AE | 1015 | (98) | 548 | (99) | 445 | (98) |
| Blood and Lymphatic | ||||||
| Anemia | 344 | (33) | 175 | (32) | 128 | (28) |
| Neutropenia | 124 | (12) | 83 | (15) | 35 | (8) |
| Thrombocytopenia | 102 | (10) | 53 | (10) | 20 | (4) |
| Gastrointestinal | ||||||
| Nausea | 476 | (46) | 266 | (48) | 171 | (38) |
| Vomiting | 333 | (32) | 183 | (33) | 122 | (27) |
| Constipation | 320 | (31) | 162 | (29) | 174 | (38) |
| Diarrhea | 249 | (24) | 162 | (29) | 83 | (18) |
| Abdominal Pain | 143 | (14) | 81 | (15) | 48 | (11) |
| Dyspepsia | 105 | (10) | 74 | (13) | 31 | (7) |
| Stomatitis | 86 | (8) | 65 | (12) | 14 | (3) |
| Sore Throat | 82 | (8) | 61 | (11) | 17 | (4) |
| General Disorders and Administration Site | ||||||
| Fatigue | 398 | (39) | 240 | (43) | 130 | (29) |
| Pyrexia | 328 | (32) | 172 | (31) | 89 | (20) |
| Weakness | 252 | (24) | 108 | (19) | 114 | (25) |
| Edema Lower Limb | 215 | (21) | 126 | (23) | 84 | (19) |
| Rigors | 112 | (11) | 62 | (11) | 28 | (6) |
| Infections | ||||||
| Urinary Tract Infection | 124 | (12) | 50 | (9) | 41 | (9) |
| Upper Respiratory Tract Infection | 101 | (10) | 82 | (15) | 30 | (7) |
| Metabolism | ||||||
| Anorexia | 231 | (22) | 81 | (15) | 105 | (23) |
| Weight Decreased | 164 | (16) | 50 | (9) | 61 | (13) |
| Dehydration | 145 | (14) | 60 | (11) | 59 | (13) |
| Appetite Decreased | 130 | (13) | 48 | (9) | 45 | (10) |
| Musculoskeletal | ||||||
| Bone Pain | 569 | (55) | 316 | (57) | 284 | (62) |
| Myalgia | 239 | (23) | 143 | (26) | 74 | (16) |
| Arthralgia | 216 | (21) | 131 | (24) | 73 | (16) |
| Back Pain | 156 | (15) | 106 | (19) | 40 | (9) |
| Pain in Limb | 143 | (14) | 84 | (15) | 52 | (11) |
| Neoplasms | ||||||
| Malignant Neoplasm Aggravated | 205 | (20) | 97 | (17) | 89 | (20) |
| Nervous | ||||||
| Headache | 191 | (19) | 149 | (27) | 50 | (11) |
| Dizziness (excluding vertigo) | 180 | (18) | 91 | (16) | 58 | (13) |
| Insomnia | 166 | (16) | 111 | (20) | 73 | (16) |
| Paresthesia | 149 | (15) | 85 | (15) | 35 | (8) |
| Hypoesthesia | 127 | (12) | 65 | (12) | 43 | (10) |
| Psychiatric | ||||||
| Depression | 146 | (14) | 95 | (17) | 49 | (11) |
| Anxiety | 112 | (11) | 73 | (13) | 37 | (8) |
| Confusion | 74 | (7) | 39 | (7) | 47 | (10) |
| Respiratory | ||||||
| Dyspnea | 282 | (27) | 155 | (28) | 107 | (24) |
| Cough | 224 | (22) | 129 | (23) | 65 | (14) |
| Skin | ||||||
| Alopecia | 125 | (12) | 80 | (14) | 36 | (8) |
| Dermatitis | 114 | (11) | 74 | (13) | 38 | (8) |
Grade 3 and Grade 4 laboratory abnormalities for serum creatinine, serum calcium, serum phosphorus, and serum magnesium observed in three clinical trials of Zometa in patients with bone metastases are shown in Tables 8 and 9.
| *Serum creatinine data for all patients randomized after the 15-minute infusion amendment. 1Grade 3 (greater than 3x Upper Limit of Normal); Grade 4 (greater than 6x Upper Limit of Normal). 2Grade 3 (less than 7 mg/dL); Grade 4 (less than 6 mg/dL). 3Grade 3 (less than 2 mg/dL); Grade 4 (less than 1 mg/dL). 4Grade 3 (greater than 3 mEq/L); Grade 4 (greater than 8 mEq/L). 5Grade 3 (less than 0.9 mEq/L); Grade 4 (less than 0.7 mEq/L). |
||||||
| Grade 3 | ||||||
| Laboratory Parameter | Zometa | Pamidronate | Placebo | |||
| 4 mg | 90 mg | |||||
| n/N | (%) | n/N | (%) | n/N | (%) | |
| Serum Creatinine1* | 7/529 | (1%) | 4/268 | (2%) | 4/241 | (2%) |
| Hypocalcemia2 | 6/973 | (< 1%) | 4/536 | (< 1%) | 0/415 | 0 |
| Hypophosphatemia3 | 115/973 | (12%) | 38/537 | (7%) | 14/415 | (3%) |
| Hypermagnesemia4 | 19/971 | (2%) | 2/535 | (< 1%) | 8/415 | (2%) |
| Hypomagnesemia5 | 1/971 | (< 1%) | 0/535 | — | 1/415 | (< 1%) |
| *Serum creatinine data for all patients randomized after the 15-minute infusion amendment. 1Grade 3 (greater than 3x Upper Limit of Normal); Grade 4 (greater than 6x Upper Limit of Normal). 2Grade 3 (less than 7 mg/dL); Grade 4 (less than 6 mg/dL). 3Grade 3 (less than 2 mg/dL); Grade 4 (less than 1 mg/dL). 4Grade 3 (greater than 3 mEq/L); Grade 4 (greater than 8 mEq/L). 5Grade 3 (less than 0.9 mEq/L); Grade 4 (less than 0.7 mEq/L). |
||||||
| Grade 4 | ||||||
| Laboratory Parameter | Zometa | Pamidronate | Placebo | |||
| 4 mg | 90 mg | |||||
| n/N | (%) | n/N | (%) | n/N | (%) | |
| Serum Creatinine1* | 2/529 | (< 1%) | 1/268 | (< 1%) | 0/241 | 0 |
| Hypocalcemia2 | 7/973 | (< 1%) | 3/536 | (< 1%) | 2/415 | (< 1%) |
| Hypophosphatemia3 | 5/973 | (< 1%) | 0/537 | 0 | 1/415 | (< 1%) |
| Hypermagnesemia4 | 0/971 | 0 | 0/535 | 0 | 2/415 | (< 1%) |
| Hypomagnesemia5 | 2/971 | (< 1%) | 1/535 | (< 1%) | 0/415 | 0 |
Among the less frequently occurring adverse events (less than 15% of patients), rigors, hypokalemia, influenza-like illness, and hypocalcemia showed a trend for more events with bisphosphonate administration (Zometa 4 mg and pamidronate groups) compared to the placebo group.
Less common adverse events reported more often with Zometa 4 mg than pamidronate included decreased weight, which was reported in 16% of patients in the Zometa 4 mg group compared with 9% in the pamidronate group. Decreased appetite was reported in slightly more patients in the Zometa 4 mg group (13%) compared with the pamidronate (9%) and placebo (10%) groups, but the clinical significance of these small differences is not clear.
Renal Toxicity
In the bone metastases trials, renal deterioration was defined as an increase of 0.5 mg/dL for patients with normal baseline creatinine (less than 1.4 mg/dL) or an increase of 1.0 mg/dL for patients with an abnormal baseline creatinine (greater than or equal to1.4 mg/dL). The following are data on the incidence of renal deterioration in patients receiving Zometa 4 mg over 15 minutes in these trials (see Table 10).
| *Table includes only patients who were randomized to the trial after a protocol amendment that lengthened the infusion duration of Zometa to 15 minutes. | ||||
| Patient Population/Baseline Creatinine | ||||
| Multiple Myeloma and Breast Cancer | Zometa 4 mg | Pamidronate 90 mg | ||
| n/N | (%) | n/N | (%) | |
| Normal | 27/246 | (11%) | 23/246 | (9%) |
| Abnormal | 2/26 | (8%) | 2/22 | (9%) |
| Total | 29/272 | (11%) | 25/268 | (9%) |
| Solid Tumors | Zometa 4 mg | Placebo | ||
| n/N | (%) | n/N | (%) | |
| Normal | 17/154 | (11%) | 10/143 | (7%) |
| Abnormal | 1/11 | (9%) | 1/20 | (5%) |
| Total | 18/165 | (11%) | 11/163 | (7%) |
| Prostate Cancer | Zometa 4 mg | Placebo | ||
| n/N | (%) | n/N | (%) | |
| Normal | 12/82 | (15%) | 8/68 | (12%) |
| Abnormal | 4/10 | (40%) | 2/10 | (20%) |
| Total | 16/92 | (17%) | 10/78 | (13%) |
The risk of deterioration in renal function appeared to be related to time on study, whether patients were receiving Zometa (4 mg over 15 minutes), placebo, or pamidronate.
In the trials and in postmarketing experience, renal deterioration, progression to renal failure, and dialysis have occurred in patients with normal and abnormal baseline renal function, including patients treated with 4 mg infused over a 15-minute period. There have been instances of this occurring after the initial Zometa dose.
6.2 Postmarketing Experience
The following adverse reactions have been reported during postapproval use of Zometa. Because these reports are from a population of uncertain size and are subject to confounding factors, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Osteonecrosis of the Jaw
Cases of osteonecrosis (primarily involving the jaw but also of other anatomical sites including hip, femur and external auditory canal) have been reported predominantly in cancer patients treated with intravenous bisphosphonates including Zometa. Many of these patients were also receiving chemotherapy and corticosteroids which may be a risk factor for ONJ. Caution is advised when Zometa is administered with anti-angiogenic drugs as an increased incidence of ONJ has been observed with concomitant use of these drugs. Data suggests a greater frequency of reports of ONJ in certain cancers, such as advanced breast cancer and multiple myeloma. The majority of the reported cases are in cancer patients following invasive dental procedures, such as tooth extraction. It is therefore prudent to avoid invasive dental procedures as recovery may be prolonged [see Warnings and Precautions (5.4)].
Acute Phase Reaction
Within three days after Zometa administration, an acute phase reaction has been reported, with symptoms including pyrexia, fatigue, bone pain and/or arthralgias, myalgias, chills, influenza-like illness and arthritis with subsequent joint swelling; these symptoms usually resolve within three days of onset, but resolution could take up to 7 to 14 days. However, some of these symptoms have been reported to persist for a longer duration.
Musculoskeletal Pain
Severe and occasionally incapacitating bone, joint, and/or muscle pain has been reported with bisphosphonate use [see Warnings and Precautions (5.5)].
Atypical Subtrochanteric and Diaphyseal Femoral Fractures
Atypical subtrochanteric and diaphyseal femoral fractures have been reported with bisphosphonate therapy, including Zometa [see Warnings and Precautions (5.6)].
Ocular Adverse Events
Cases of uveitis, scleritis, episcleritis, conjunctivitis, iritis, and orbital inflammation including orbital edema have been reported during postmarketing use. In some cases, symptoms resolved with topical steroids.
Hypersensitivity Reactions
There have been rare reports of allergic reaction with intravenous zoledronic acid including angioedema and bronchoconstriction. Very rare cases of anaphylactic reaction/shock have been reported. Cases of Stevens-Johnson syndrome and toxic epidermal necrolysis have also been reported.
Additional adverse reactions reported in postmarketing use include:
CNS: taste disturbance, hyperesthesia, tremor; Special Senses: blurred vision; uveitis; Gastrointestinal: dry mouth; Skin: Increased sweating; Musculoskeletal: muscle cramps; Cardiovascular: hypertension, bradycardia, hypotension (associated with syncope or circulatory collapse primarily in patients with underlying risk factors); Respiratory: bronchospasms, interstitial lung disease (ILD) with positive rechallenge; Renal: hematuria, proteinuria, acquired Fanconi syndrome; General Disorders and Administration Site: weight increase, influenza-like illness (pyrexia, asthenia, fatigue or malaise) persisting for greater than 30 days; Laboratory Abnormalities: hyperkalemia, hypernatremia, hypocalcemia (cardiac arrhythmias and neurologic adverse events including seizures, tetany, and numbness have been reported due to severe hypocalcemia).
7. Drug Interactions
In vitro studies indicate that the plasma protein binding of zoledronic acid is low, with the unbound fraction ranging from 60%-77%. In vitro studies also indicate that zoledronic acid does not inhibit microsomal CYP450 enzymes. In vivo studies showed that zoledronic acid is not metabolized, and is excreted into the urine as the intact drug.
7.1 Aminoglycosides and Calcitonin
Caution is advised when bisphosphonates are administered with aminoglycosides or calcitonin, since these agents may have an additive effect to lower serum calcium level for prolonged periods. This effect has not been reported in Zometa clinical trials.
7.2 Loop Diuretics
Caution should also be exercised when Zometa is used in combination with loop diuretics due to an increased risk of hypocalcemia.
7.3 Nephrotoxic Drugs
Caution is indicated when Zometa is used with other potentially nephrotoxic drugs.
7.4 Thalidomide
No dose adjustment for Zometa 4 mg is needed when coadministered with thalidomide. In a pharmacokinetic study of 24 patients with multiple myeloma, Zometa 4 mg given as a 15-minute infusion was administered either alone or with thalidomide (100 mg once daily on days 1-14 and 200 mg once daily on Days 15-28). Coadministration of thalidomide with Zometa did not significantly change the pharmacokinetics of zoledronic acid or creatinine clearance.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action, Zometa can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data in pregnant women to inform the drug-associated risk. In animal reproduction studies, administration of zoledronic acid to pregnant rats during organogenesis resulted in fetal malformations and embryo-fetal lethality at maternal exposures that were ≥ 2.4 times the human clinical exposure based on AUC (see Data). Bisphosphonates, such as Zometa, are incorporated into the bone matrix, from where they are gradually released over periods of weeks to years. There may be a risk of fetal harm (e.g., skeletal and other abnormalities) if a woman becomes pregnant after completing a course of bisphosphonate therapy. Advise pregnant women and females of reproductive potential of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown; however, in the U.S. general population, the estimated background risk of major birth defects is 2%-4% and of miscarriage is 15%-20% of clinically recognized pregnancies.
Data
Animal Data
In female rats given subcutaneous doses of zoledronic acid of 0.01, 0.03, or 0.1 mg/kg/day beginning 15 days before mating and continuing through gestation, the number of stillbirths was increased and survival of neonates was decreased in the mid- and high-dose groups (greater than or equal to 0.2 times the human systemic exposure following an intravenous dose of 4 mg, based on an AUC comparison). Adverse maternal effects were observed in all dose groups (with a systemic exposure of greater than or equal to 0.07 times the human systemic exposure following an intravenous dose of 4 mg, based on an AUC comparison) and included dystocia and periparturient mortality in pregnant rats allowed to deliver. Maternal mortality may have been related to drug-induced inhibition of skeletal calcium mobilization, resulting in periparturient hypocalcemia. This appears to be a bisphosphonate-class effect.
In pregnant rats given a subcutaneous dose of zoledronic acid of 0.1, 0.2, or 0.4 mg/kg/day during gestation, adverse fetal effects were observed in the mid- and high-dose groups (with systemic exposures of 2.4 and 4.8 times, respectively, the human systemic exposure following an intravenous dose of 4 mg, based on an AUC comparison). These adverse effects included increases in pre- and postimplantation losses, decreases in viable fetuses, and fetal skeletal, visceral, and external malformations. Fetal skeletal effects observed in the high-dose group included unossified or incompletely ossified bones, thickened, curved, or shortened bones, wavy ribs, and shortened jaw. Other adverse fetal effects observed in the high-dose group included reduced lens, rudimentary cerebellum, reduction or absence of liver lobes, reduction of lung lobes, vessel dilation, cleft palate, and edema. Skeletal variations were also observed in the low dose group at 0.1 mg/kg/day (with systemic exposure of 1.2 times the human systemic exposure following an intravenous dose of 4 mg, based on an AUC comparison). Signs of maternal toxicity were observed in the high-dose group and included reduced body weights and food consumption, indicating that maximal exposure levels were achieved in this study.
In pregnant rabbits given subcutaneous doses of zoledronic acid of 0.01, 0.03, or 0.1 mg/kg/day during gestation (less than or equal to 0.5 times the human intravenous dose of 4 mg, based on a comparison of relative body surface areas), no adverse fetal effects were observed. Maternal mortality and abortion occurred in all treatment groups (at doses greater than or equal to 0.05 times the human intravenous dose of 4 mg, based on a comparison of relative body surface areas). Adverse maternal effects were associated with, and may have been caused by, drug-induced hypocalcemia.
8.2 Lactation
Risk Summary
After administration of Zometa, it is not known whether zoledronic acid is present in human milk, or whether it affects milk production or the breastfed child. Zoledronic acid binds to bone long term and may be released over periods of weeks to years. Because of the potential for serious adverse reactions in a breastfed child, advise a lactating woman not to breastfeed during and after Zometa treatment.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status of females of reproductive potential prior to initiation of Zometa.
Contraception
Females
Zometa can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Zoledronic acid binds to bone long term and may be released over periods of weeks to years. Advise females of reproductive potential to use effective contraception during and after Zometa treatment.
Infertility
Females
Based on animal studies, Zometa may impair fertility in females of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Zometa is not indicated for use in children.
The safety and effectiveness of zoledronic acid was studied in a one-year, active-controlled trial of 152 pediatric subjects (74 receiving zoledronic acid). The enrolled population was subjects with severe osteogenesis imperfecta, aged 1-17 years, 55% male, 84% Caucasian, with a mean lumbar spine bone mineral density (BMD) of 0.431 gm/cm2, which is 2.7 standard deviations below the mean for age-matched controls (BMD Z-score of -2.7). At one year, increases in BMD were observed in the zoledronic acid treatment group. However, changes in BMD in individual patients with severe osteogenesis imperfecta did not necessarily correlate with the risk for fracture or the incidence or severity of chronic bone pain. The adverse events observed with Zometa use in children did not raise any new safety findings beyond those previously seen in adults treated for hypercalcemia of malignancy or bone metastases. However, adverse reactions seen more commonly in pediatric patients included pyrexia (61%), arthralgia (26%), hypocalcemia (22%) and headache (22%). These reactions, excluding arthralgia, occurred most frequently within 3 days after the first infusion and became less common with repeat dosing. Because of long-term retention in bone, Zometa should only be used in children if the potential benefit outweighs the potential risk.
Plasma zoledronic acid concentration data was obtained from 10 patients with severe osteogenesis imperfecta (4 in the age group of 3-8 years and 6 in the age group of 9-17 years) infused with 0.05 mg/kg dose over 30 min. Mean Cmax and AUC(0-last) was 167 ng/mL and 220 ng•h/mL, respectively. The plasma concentration time profile of zoledronic acid in pediatric patients represent a multi-exponential decline, as observed in adult cancer patients at an approximately equivalent mg/kg dose.
8.5 Geriatric Use
Clinical studies of Zometa in hypercalcemia of malignancy included 34 patients who were 65 years of age or older. No significant differences in response rate or adverse reactions were seen in geriatric patients receiving Zometa as compared to younger patients. Controlled clinical studies of Zometa in the treatment of multiple myeloma and bone metastases of solid tumors in patients over age 65 revealed similar efficacy and safety in older and younger patients. Because decreased renal function occurs more commonly in the elderly, special care should be taken to monitor renal function.
| ZOMETA
zoledronic acid injection, solution, concentrate |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Novartis Pharmaceuticals Corporation (002147023) |