Drug Detail:Tezspire (Tezepelumab-ekko)
Generic Name: TEZEPELUMAB 210mg in 1.9mL
Dosage Form: injection, solution
Drug Class: Selective immunosuppressants
Recommended Dosage
The recommended dosage of TEZSPIRE is 210 mg administered subcutaneously once every 4 weeks.
Missed Dose Information
If a dose is missed, administer the dose as soon as possible. Thereafter, the patient can continue (resume) dosing on the usual day of administration. If the next dose is already due, then administer as planned.
Preparation and Administration Instructions
TEZSPIRE vial and pre‑filled syringe are intended for administration by a healthcare provider.
TEZSPIRE pre-filled pen can be administered by patients/caregivers or healthcare providers. Patients/caregivers may administer TEZSPIRE pre-filled pen after proper training in subcutaneous injection technique and after the healthcare provider determines it is appropriate.
Each vial, pre-filled syringe and pre‑filled pen contain a single dose of TEZSPIRE.
- •
- Prior to administration, remove TEZSPIRE from the refrigerator and allow it to reach room temperature. This generally takes 60 minutes. Do not expose to heat and do not shake. Do not use if the security seal on the carton has been broken. Do not put back in the refrigerator once TEZSPIRE has reached room temperature.
- •
- Visually inspect TEZSPIRE for particulate matter and discoloration prior to administration. TEZSPIRE is a clear to opalescent, colorless to light yellow solution. Do not use TEZSPIRE if liquid is cloudy, discolored, or if it contains large particles or foreign particulate matter. Do not use if the vial, pre-filled syringe or pre‑filled pen has been dropped or damaged or if the expiration date has passed.
- •
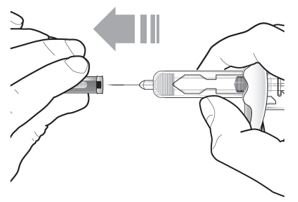
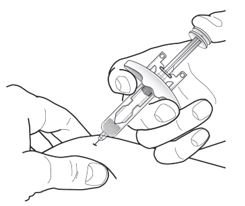
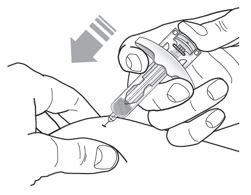
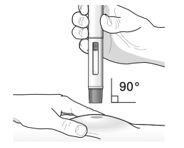
- Inject TEZSPIRE 210 mg (contents of one vial, one pre-filled syringe or one pre-filled pen as described below) subcutaneously into the thigh or abdomen, except for the 2 inches (5 cm) around the navel. If a healthcare provider or caregiver administers the injection, the upper arm can also be used. A patient should not self-inject in the upper arm. TEZSPIRE should not be injected into areas where the skin is tender, bruised, erythematous, or hardened. It is recommended to rotate the injection site with each injection.
Administration Instructions for Single-Dose Pre-filled Syringe
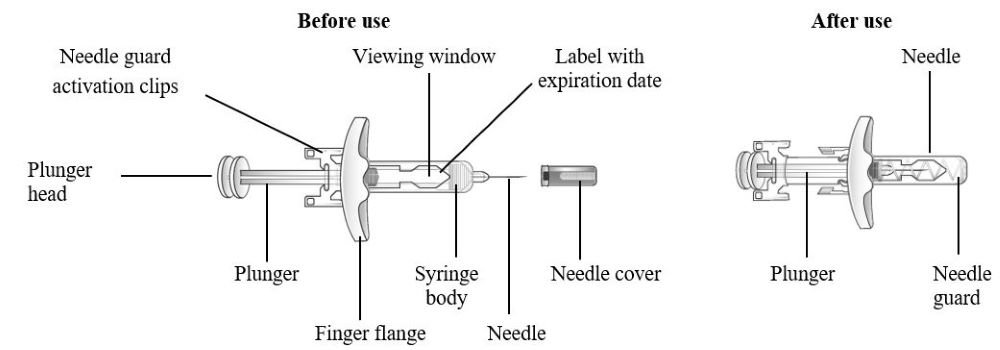
Refer to Figure 1 to identify the pre-filled syringe components for use in the administration steps.
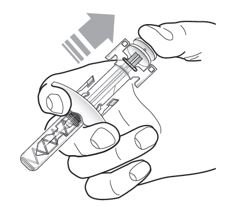
Do not remove the needle cover until Step 2 of these instructions when you are ready to inject TEZSPIRE. Do not touch the needle guard activation clips to prevent premature activation of the needle safety guard.
Figure 1 TEZSPIRE Pre-filled Syringe Components
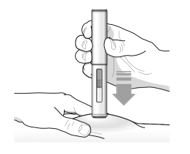
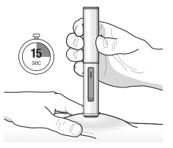
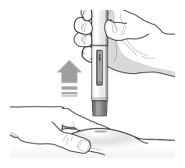
Administration Instructions for Single-Dose Pre-filled Pen
These administration instructions are intended for healthcare providers use only. Patients and caregivers should refer to the TEZSPIRE pre-filled pen ‘Instructions for Use’ for more detailed instructions on the preparation and administration of TEZSPIRE pre-filled pen [See Instructions for Use].
Patients/caregivers may inject after proper training in subcutaneous injection technique according to the ‘Instructions for Use’, and after the healthcare provider determines it is appropriate.
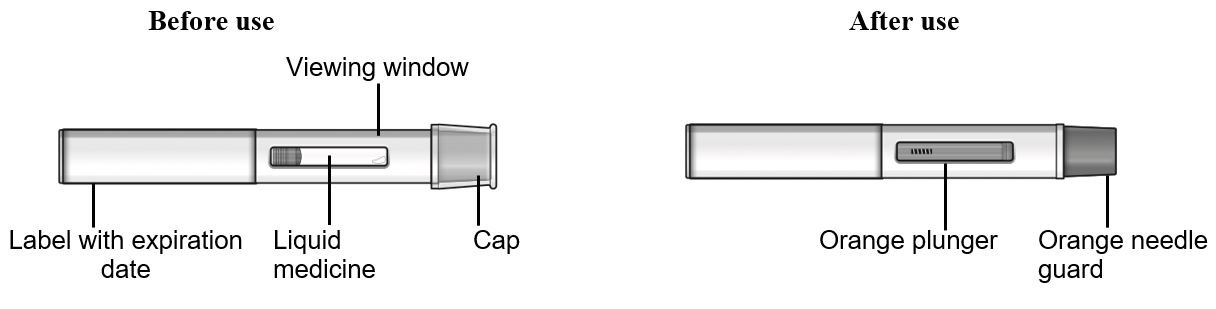
Refer to Figure 2 to identify the pre-filled pen components for use in the administration steps.
Do not remove the cap until you are ready to inject TEZSPIRE.
Figure 2 TEZSPIRE Pre-filled Pen Components