Drug Detail:Asacol hd (Mesalamine (oral) [ me-sal-a-meen ])
Drug Class: 5-aminosalicylates
Highlights of Prescribing Information
ASACOL® HD (mesalamine) delayed-release tablets, for oral use
Initial U.S. Approval: 1987
Recent Major Changes
- Warnings and Precautions
Renal Impairment (5.1) 11/2022
Indications and Usage for Asacol HD
Asacol HD is an aminosalicylate indicated for the treatment of moderately active ulcerative colitis in adults. (1)
Limitation of Use:
Safety and effectiveness of Asacol HD beyond 6 weeks have not been established (1)
Asacol HD Dosage and Administration
Important Administration Instructions:
- Do not substitute one Asacol HD 800 tablet for two mesalamine delayed-release 400 mg oral products. (2.1)
- Evaluate renal function prior to initiation of Asacol HD. (2.1, 5.1)
- Take on an empty stomach, at least 1 hour before and 2 hours after a meal. (2.1)
- Swallow whole; do not cut, break or chew the tablets. (2.1)
- Drink an adequate amount of fluids. (2.1, 5.7)
Treatment of Moderately Active Ulcerative Colitis:
- Recommended dosage is 1600 mg (two 800 mg tablets) three times daily for 6 weeks. (2.2)
Dosage Forms and Strengths
Delayed-release tablets: 800 mg (3)
Contraindications
Known or suspected hypersensitivity to salicylates or aminosalicylates or to any of the ingredients of Asacol HD tablets. (4, 5.3)
Warnings and Precautions
-
Renal Impairment: Assess renal function at the beginning of treatment and periodically during treatment. Evaluate the risks and benefits in patients with known renal impairment or taking nephrotoxic drugs; monitor renal function. Discontinue Asacol HD if renal function deteriorates. (5.1, 7.1, 8.6)
-
Mesalamine-induced Acute Intolerance Syndrome: Symptoms may be difficult to distinguish from an ulcerative colitis exacerbation; monitor for worsening symptoms; discontinue if acute intolerance syndrome suspected. (5.2)
-
Hypersensitivity Reactions, including Myocarditis and Pericarditis: Evaluate patients immediately and discontinue if a hypersensitivity reaction is suspected. (5.3)
-
Hepatic Failure: Evaluate the risks and benefits in patients with known liver impairment. (5.4)
-
Severe Cutaneous Adverse Reactions: Discontinue at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation. (5.5)
-
Photosensitivity: Advise patients with pre-existing skin conditions to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors. (5.6)
-
Nephrolithiasis: Mesalamine-containing stones are undetectable by standard radiography or computed tomography (CT). Ensure adequate hydration during treatment. (5.7)
-
Iron Content of Asacol HD: Consider the iron content of Asacol HD in patients taking iron supplementation and those at risk of iron overload. (5.8)
- Interference with Laboratory Tests: Use of mesalamine may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection. (5.9)
Adverse Reactions/Side Effects
The most common adverse reactions (≥2%) are headache, nausea, nasopharyngitis, abdominal pain, and worsening of ulcerative colitis (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1-800-678-1605 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Drug Interactions
-
Nephrotoxic Agents including NSAIDs: Increased risk of nephrotoxicity; monitor for changes in renal function and mesalamine-related adverse reactions. (7.1)
- Azathioprine or 6-Mercaptopurine: Increased risk of blood disorders; monitor complete blood cell counts and platelet counts. (7.2)
Use In Specific Populations
Geriatric Patients: Increased risk of blood dyscrasias; monitor complete blood cell counts and platelet counts. (8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2022
Full Prescribing Information
1. Indications and Usage for Asacol HD
Asacol HD is indicated for the treatment of moderately active ulcerative colitis in adults.
Limitations of Use:
Safety and effectiveness of Asacol HD beyond 6 weeks have not been established.
2. Asacol HD Dosage and Administration
2.1 Important Administration Instructions
- Do not substitute one Asacol HD 800 tablet for two mesalamine delayed-release 400 mg oral products [see Clinical Pharmacology (12.3)].
- Evaluate renal function prior to initiation of Asacol HD.
- Take Asacol HD tablets on an empty stomach, at least 1 hour before and 2 hours after a meal [see Clinical Pharmacology (12.3)].
- Swallow Asacol HD tablets whole. Do not cut, break or chew the tablets.
- Drink an adequate amount of fluids [see Warnings and Precautions (5.7)].
- Intact, partially intact, and/or tablet shells have been reported in the stool; Instruct patients to contact their healthcare provider if this occurs repeatedly.
- Protect Asacol HD tablets from moisture.
3. Dosage Forms and Strengths
Asacol HD delayed-release tablets: 800 mg (red-brown, capsule-shaped and imprinted with “WC 800” in black).
4. Contraindications
Asacol HD is contraindicated in patients with known or suspected hypersensitivity to salicylates or aminosalicylates or to any of the ingredients of Asacol HD [see Warnings and Precautions (5.3), Adverse Reactions (6.2), and Description (11)].
5. Warnings and Precautions
5.1 Renal Impairment
Renal impairment, including minimal change disease, acute and chronic interstitial nephritis, and, rarely, renal failure, has been reported in patients taking products such as Asacol HD that contain or are converted to mesalamine [see Adverse Reactions (6.2)]. In animal studies, the kidney was the principal organ of mesalamine toxicity [see Adverse Reactions (6.2), Nonclinical Toxicology (13.2)].
Evaluate renal function prior to initiation of Asacol HD and periodically while on therapy. Evaluate the risks and benefits of using Asacol HD in patients with known renal impairment or history of renal disease or taking concomitant nephrotoxic drugs. Discontinue Asacol HD if renal function deteriorates while on therapy [see Drug Interactions (7.1), Use in Specific Populations (8.6)].
5.2 Mesalamine-Induced Acute Intolerance Syndrome
Mesalamine has been associated with an acute intolerance syndrome that may be difficult to distinguish from an exacerbation of ulcerative colitis. Exacerbation of the symptoms of colitis has been reported in 2.3% of Asacol HD-treated patients in controlled clinical trials. This acute reaction, characterized by cramping, abdominal pain, bloody diarrhea, and occasionally by fever, headache, malaise, pruritus, rash, and conjunctivitis, has been reported after the initiation of Asacol HD tablets as well as other mesalamine products. Symptoms usually abate when Asacol HD tablets are discontinued. Monitor patients for worsening of these symptoms while on treatment. If acute intolerance syndrome is suspected, promptly discontinue treatment with Asacol HD.
5.3 Hypersensitivity Reactions
Hypersensitivity reactions have been reported in patients taking sulfasalazine. Some patients may have a similar reaction to Asacol HD tablets or to other compounds that contain or are converted to mesalamine.
As with sulfasalazine, mesalamine-induced hypersensitivity reactions may present as internal organ involvement, including myocarditis, pericarditis, nephritis, hepatitis, pneumonitis, and hematologic abnormalities. Evaluate patients immediately if signs or symptoms of a hypersensitivity reaction are present. Discontinue Asacol HD if an alternative etiology for the signs or symptoms cannot be established.
5.4 Hepatic Failure
There have been reports of hepatic failure in patients with pre-existing liver disease who have been administered mesalamine. Evaluate the risks and benefits of using Asacol HD in patients with known liver impairment.
5.5 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported with use of mesalamine [see Adverse Reactions (6.2)]. Discontinue Asacol HD at the first appearance of signs or symptoms of severe cutaneous adverse reactions, or other signs of hypersensitivity and consider further evaluation.
5.6 Photosensitivity
Patients treated with mesalamine or sulfasalazine who have pre-existing skin conditions such as atopic dermatitis and atopic eczema have reported more severe photosensitivity reactions. Advise patients to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors.
5.7 Nephrolithiasis
Cases of nephrolithiasis have been reported with the use of mesalamine, including stones of 100% mesalamine content. Mesalamine-containing stones are radiotransparent and undetectable by standard radiography or computed tomography (CT). Ensure adequate fluid intake during treatment with Asacol HD.
5.8 Iron Content of Asacol HD
Asacol HD contains iron oxide as a colorant in the coating of the delayed-release tablets. Each 800 mg delayed-release tablet contains 4.9 mg of iron. The total content of iron is 29.2 mg at the recommended daily dosage [see Dosage and Administration (2.2)]. Before prescribing Asacol HD to patients receiving iron supplementation or those at risk for developing iron overload, consider the combined daily amount of iron from all sources, including Asacol HD.
5.9 Interference with Laboratory Tests
Use of Asacol HD may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection because of the similarity in the chromatograms of normetanephrine and the main metabolite of mesalamine, N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA). Consider an alternative, selective assay for normetanephrine.
6. Adverse Reactions/Side Effects
The following serious or clinically significant adverse described elsewhere in labeling are:
- Renal Impairment [see Warnings and Precautions (5.1)]
- Mesalamine-Induced Acute Intolerance Syndrome [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Hepatic Failure [see Warnings and Precautions (5.4)]
- Severe Cutaneous Adverse Reactions [see Warnings and Precautions (5.5)]
- Photosensitivity [see Warnings and Precautions (5.6)]
- Nephrolithiasis [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Asacol HD has been evaluated in 896 patients with ulcerative colitis in controlled studies. Three six- week, active-controlled studies were conducted comparing Asacol HD 4.8 grams per day with mesalamine-delayed release tablets 2.4 grams per day in patients with mildly to moderately active ulcerative colitis. In these studies, 727 patients were dosed with Asacol HD tablets and 732 patients were dosed with mesalamine delayed-release tablets.
The most common reactions reported in the Asacol HD group were headache (4.7%), nausea (2.8%), nasopharyngitis (2.5%), abdominal pain (2.3%), diarrhea (1.7%), and dyspepsia (1.7%); Table 1 enumerates adverse reactions that occurred in the three studies. The most common reactions in patients with moderately active ulcerative colitis (602 patients dosed with Asacol HD and 618 patients dosed with mesalamine delayed-release 400 mg) were the same as all treated patients.
Discontinuations due to adverse reactions occurred in 3.9% of patients in the Asacol HD group and in 4.2% of patients in the mesalamine delayed-release tablet comparator group. The most common cause for discontinuation was gastrointestinal symptoms associated with ulcerative colitis.
Serious adverse reactions occurred in 0.8% of patients in the Asacol HD group and in 1.8% of patients in the mesalamine delayed-release tablet comparator group. The majority involved the gastrointestinal system.
| Table 1. Adverse Reactions Occurring in ≥1% of All Treated Patients (Three studies combined)
|
||
| Mesalamine delayed-release 2.4 grams per day | Asacol HD 4.8 grams per day |
|
| (400 mg Tablet) | (800 mg Tablet) | |
| Adverse Reaction | (N = 732) | (N = 727) |
| Headache | 4.9 % | 4.7 % |
| Nausea | 2.9 % | 2.8 % |
| Nasopharyngitis | 1.4 % | 2.5 % |
| Abdominal pain | 2.3 % | 2.3 % |
| Diarrhea | 1.9 % | 1.7 % |
| Dyspepsia | 0.8 % | 1.7 % |
| Vomiting | 1.6 % | 1.4 % |
| Flatulence | 0.7 % | 1.2 % |
| Influenza | 1.2 % | 1.0 % |
| Pyrexia | 1.2 % | 0.7 % |
| Cough | 1.4 % | 0.3 % |
| N = number of patients within specified treatment group | ||
| Percent = percentage of patients in category and treatment group | ||
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Asacol HD or other mesalamine-containing products or products that are metabolized to mesalamine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: Facial edema, edema, peripheral edema, asthenia, chills, infection, malaise, pain, neck pain, chest pain, back pain, abdominal enlargement, lupus-like syndrome, drug fever (rare).
Cardiovascular: Pericarditis (rare) and myocarditis (rare) [see Warnings and Precautions (5.3)], pericardial effusion, vasodilation, migraine.
Endocrine: Nephrogenic diabetes insipidus.
Gastrointestinal: Dry mouth, stomatitis, oral ulcers, anorexia, increased appetite, eructation, pancreatitis, cholecystitis, gastritis, gastroenteritis, gastrointestinal bleeding, perforated peptic ulcer (rare), constipation, hemorrhoids, rectal hemorrhage, bloody diarrhea, tenesmus, stool abnormality.
Hepatic: There have been rare reports of hepatotoxicity, including jaundice, cholestatic jaundice, hepatitis, and possible hepatocellular damage including liver necrosis and liver failure. Some of these cases were fatal. Asymptomatic elevations of liver enzymes which usually resolve during continued use or with discontinuation of the drug have also been reported. One case of Kawasaki-like syndrome, that included changes in liver enzymes, was also reported [see Warnings and Precautions (5.4)].
Hematologic: Agranulocytosis (rare), aplastic anemia (rare), anemia, thrombocytopenia, leukopenia, eosinophilia, lymphadenopathy.
Musculoskeletal: Gout, rheumatoid arthritis, arthritis, arthralgia, joint disorder, myalgia, hypertonia.
Neurological/Psychiatric: Anxiety, depression, somnolence, insomnia, nervousness, confusion, emotional lability, dizziness, vertigo, tremor, paresthesia, hyperesthesia, peripheral neuropathy (rare), Guillain-Barré syndrome (rare), transverse myelitis (rare), and intracranial hypertension.
Respiratory/Pulmonary: Sinusitis, rhinitis, pharyngitis, asthma exacerbation, pleuritis/pleurisy, bronchitis, eosinophilic pneumonia, interstitial pneumonitis.
Skin: Alopecia, psoriasis (rare), pyoderma gangrenosum (rare), erythema nodosum, acne, dry skin, sweating, pruritus, urticaria, rash, SJS/TEN, DRESS, and AGEP [see Warnings and Precautions (5.5)].
Special Senses: Ear pain, tinnitus, ear congestion, ear disorder, conjunctivitis, eye pain, blurred vision, vision abnormality, taste perversion.
Renal/Urogenital: Renal failure (rare), interstitial nephritis, minimal change disease, nephrolithiasis [see Warnings and Precautions (5.1, 5.7)], dysuria, urinary frequency and urgency, hematuria, epididymitis, decreased libido, dysmenorrhea, menorrhagia.
Laboratory Abnormalities: Elevated AST (SGOT) or ALT (SGPT), elevated alkaline phosphatase, elevated GGT, elevated LDH, elevated bilirubin, elevated serum creatinine and BUN.
7. Drug Interactions
7.1 Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs
The concurrent use of mesalamine with known nephrotoxic agents, including nonsteroidal anti-inflammatory drugs (NSAIDs) may increase the risk of nephrotoxicity. Monitor patients taking nephrotoxic drugs for changes in renal function and mesalamine-related adverse reactions [see Warnings and Precautions (5.1)].
7.2 Azathioprine or 6-Mercaptopurine
The concurrent use of mesalamine with azathioprine or 6-mercaptopurine and/or other drugs known to cause myelotoxicity may increase the risk for blood disorders, bone marrow failure, and associated complications. If concomitant use of Asacol HD and azathioprine or 6-mercaptopurine cannot be avoided, monitor blood tests, including complete blood cell counts and platelet counts.
8. Use In Specific Populations
8.5 Geriatric Use
Clinical studies of Asacol HD did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently than younger patients. Reports from uncontrolled clinical studies and postmarketing reporting systems suggested a higher incidence of blood dyscrasias (i.e., agranulocytosis, neutropenia, and pancytopenia) in patients who were 65 years or older compared to younger patients taking mesalamine-containing products such as Asacol HD. Monitor complete blood cell counts and platelet counts in elderly patients during therapy with Asacol HD.
In general, consider the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients when prescribing Asacol HD [see Use in Specific Populations (8.6)].
8.6 Renal Impairment
Mesalamine is known to be substantially excreted by the kidney, and the risk of adverse reactions may be greater in patients with impaired renal function. Evaluate renal function in all patients prior to initiation and periodically while on Asacol HD therapy. Monitor patients with known renal impairment or history of renal disease or taking nephrotoxic drugs for decreased renal function and mesalamine-related adverse reactions. Discontinue Asacol HD if renal function deteriorates while on therapy [see Warnings and Precautions (5.1), Adverse Reactions (6.2), Drug Interactions (7.1)].
11. Asacol HD Description
Each Asacol HD delayed-release tablet for oral administration contains 800 mg of mesalamine, an aminosalicylate. Asacol HD delayed-release tablets have an outer protective coat consisting of a combination of acrylic based resins, Eudragit S (methacrylic acid and methyl methacrylate copolymer (1:2), NF) and Eudragit L (methacrylic acid and methyl methacrylate copolymer (1:1), NF). The inner coat consists of an acrylic based resin, Eudragit S, which dissolves at pH 7 or greater, releasing mesalamine in the terminal ileum and beyond for topical anti-inflammatory action in the colon. Mesalamine (also referred to as 5-aminosalicylic acid or 5-ASA) has the chemical name 5-amino-2-hydroxybenzoic acid; its structural formula is:
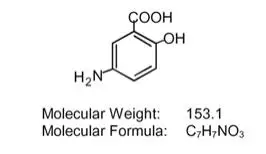
Inactive Ingredients: Each tablet contains colloidal silicon dioxide, dibutyl sebacate, edible black ink, ferric oxide red (6.15 mg), ferric oxide yellow (1.10 mg), lactose monohydrate, magnesium stearate, methacrylic acid and methyl methacrylate copolymer (1:2) (Eudragit S), methacrylic acid and methyl methacrylate copolymer (1:1) (Eudragit L), polyethylene glycol, povidone, sodium starch glycolate, and talc.
12. Asacol HD - Clinical Pharmacology
12.3 Pharmacokinetics
Absorption
Plasma concentrations of mesalamine (5-aminosalicylic acid; 5-ASA) and its metabolite, N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA) are highly variable following administration of Asacol HD tablets. Following single dose oral administration of Asacol HD 800 mg tablet in healthy subjects (N = 139) under fasted conditions, the mean Cmax, AUC8-48h and AUC0-tldc values were 208 ng/mL, 2296 ng.h/mL, and 2533 ng.h/mL, respectively. The median [range] Tmax for mesalamine following administration of Asacol HD 800 mg tablet was approximately 24 hours [4 to 72 hours], reflecting the delayed-release characteristics of the formulation.
Based on cumulative urinary recovery of mesalamine and N-Ac-5-ASA from single dose studies in healthy subjects, approximately 20% of the orally administered mesalamine in Asacol HD tablets is systemically absorbed.
Food Effect: A high calorie (800 to 1000 calories), high fat (approximately 50 % of total caloric content) meal increased mesalamine Cmax by 2.4-fold and mesalamine systemic exposure (AUC8-48 and AUC0-tldc) by 2.8-fold; the median lag-time increased by 8 hours and median tmax by 6 hours (from 24 to 30 hours) [see Dosage and Administration (2.1)].
Comparative exposure between one Asacol HD 800 mg tablet and two mesalamine delayed-release 400 mg oral products is unknown [see Dosage and Administration (2.1)].
Elimination
Metabolism
The absorbed mesalamine is acetylated in the gut mucosal wall and by the liver to N-Ac-5-ASA.
Excretion
Absorbed mesalamine is excreted mainly by the kidneys as N-acetyl-5-aminosalicylic acid. Unabsorbed mesalamine is excreted in feces.
13. Nonclinical Toxicology
13.2 Animal Toxicology and/or Pharmacology
In animal studies (rats, mice, dogs), the kidney was the principal organ for toxicity. (In the following, comparisons of animal dosing to recommended human dosing are based on body surface area and a 4.8 grams per day dose for a 60 kg person.)
Mesalamine causes renal papillary necrosis in rats at single doses of approximately 750 mg/kg to 1000 mg/kg (1.5 to 2.0 times the recommended human dose). Doses of 170 and 360 mg/kg/day (about 0.3 and 0.73 times the recommended human dose) given to rats for six months produced papillary necrosis, papillary edema, tubular degeneration, tubular mineralization, and urothelial hyperplasia.
In mice, oral doses of 4000 mg/kg/day (approximately 4.1 times the recommended human dose) for three months produced tubular nephrosis, multifocal/diffuse tubulo-interstitial inflammation, and multifocal/diffuse papillary necrosis.
In dogs, single doses of 6000 mg (approximately 6.25 times the recommended human dose) of delayed- release mesalamine tablets resulted in renal papillary necrosis but were not fatal. Renal changes have occurred in dogs given chronic administration of mesalamine at doses of 80 mg/kg/day (0.5 times the recommended human dose).
| ASACOL HD
mesalamine tablet, delayed release |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Allergan, Inc. (144796497) |





