Drug Detail:Dayvigo (Lemborexant)
Drug Class: Miscellaneous anxiolytics, sedatives and hypnotics
Highlights of Prescribing Information
DAYVIGO® (lemborexant) tablets, for oral use, CIV
Initial U.S. Approval: 2019
Recent Major Changes
Warnings and Precautions (5.4) 04/2023
Indications and Usage for Dayvigo
DAYVIGO is an orexin receptor antagonist indicated for the treatment of adult patients with insomnia, characterized by difficulties with sleep onset and/or sleep maintenance. (1)
Dayvigo Dosage and Administration
- Recommended dose is 5 mg taken no more than once per night, immediately before going to bed, with at least 7 hours remaining before the planned time of awakening. Dosage may be increased to 10 mg based on clinical response and tolerability. (2.1)
- The maximum recommended dose is 10 mg once daily. (2.1)
- Time to sleep onset may be delayed if taken with or soon after a meal. (2.1)
- Hepatic Impairment: (2.3)
○ Moderate hepatic impairment: Initial and maximum recommended dosage is 5 mg no more than once per night.
○ Severe hepatic impairment: Not recommended.
Dosage Forms and Strengths
Tablets: 5 mg, 10 mg (3)
Contraindications
DAYVIGO is contraindicated in patients with narcolepsy. (4)
Warnings and Precautions
- CNS Depressant Effects and Daytime Impairment: Impairs alertness and motor coordination including morning impairment. Risk increases with dose and use with other central nervous system (CNS) depressants. For patients taking DAYVIGO 10 mg, caution against next-day driving and other activities requiring complete mental alertness. (5.1)
- Sleep Paralysis, Hypnagogic/Hypnopompic Hallucinations, and Cataplexy-like Symptoms: May occur with use of DAYVIGO. (5.2)
- Complex Sleep Behaviors: Behaviors including sleep-walking, sleep-driving, and engaging in other activities while not fully awake may occur. Discontinue immediately if a complex sleep behavior occurs. (5.3)
- Compromised Respiratory Function: Effect on respiratory function should be considered. (5.4, 8.8)
- Worsening of Depression/Suicidal Ideation: Worsening of depression or suicidal thinking may occur. Prescribe the lowest number of tablets feasible to avoid intentional overdosage. (5.5)
- Need to Evaluate for Co-morbid Diagnoses: Reevaluate if insomnia persists after 7 to 10 days of treatment. (5.6)
Adverse Reactions/Side Effects
The most common adverse reaction (reported in ≥5% of patients treated with DAYVIGO and at least twice the rate of placebo) was somnolence. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Eisai Inc. at 1-888-274-2378 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Strong or moderate CYP3A inhibitors: Avoid concomitant use. (7.1)
- Weak CYP3A inhibitors: The maximum recommended dose is 5 mg. (2.2, 7.1)
- Strong or moderate CYP3A inducers: Avoid concomitant use. (7.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 5/2023
Related/similar drugs
Belsomra, lorazepam, melatonin, zolpidem, diphenhydramine, Ativan, AmbienFull Prescribing Information
1. Indications and Usage for Dayvigo
DAYVIGO is indicated for the treatment of adult patients with insomnia, characterized by difficulties with sleep onset and/or sleep maintenance [see Clinical Studies (14.1)].
2. Dayvigo Dosage and Administration
2.1 Dosing Information
The recommended dosage of DAYVIGO is 5 mg taken no more than once per night, immediately before going to bed, with at least 7 hours remaining before the planned time of awakening. The dose may be increased to the maximum recommended dose of 10 mg based on clinical response and tolerability. Time to sleep onset may be delayed if taken with or soon after a meal [see Clinical Pharmacology (12.3)].
2.2 Dosage Recommendations for Concomitant Use with CYP3A Inhibitors or CYP3A Inducers
Co-administration with Strong or Moderate CYP3A Inhibitors
Avoid concomitant use of DAYVIGO with strong or moderate CYP3A inhibitors [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
Co-administration with Weak CYP3A Inhibitors
The maximum recommended dosage of DAYVIGO is 5 mg no more than once per night when co-administered with weak CYP3A inhibitors [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
Co-administration with Strong or Moderate CYP3A Inducers
Avoid concomitant use of DAYVIGO with strong or moderate CYP3A inducers [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
2.3 Dosage Recommendations for Patients with Hepatic Impairment
The maximum recommended dose of DAYVIGO is 5 mg no more than once per night in patients with moderate hepatic impairment [see Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
DAYVIGO is not recommended in patients with severe hepatic impairment [see Use in Specific Populations (8.7)].
3. Dosage Forms and Strengths
DAYVIGO (lemborexant) tablets are available as:
- 5 mg tablets: pale yellow, round, biconvex, film-coated tablets, and debossed with "5" on one side and "LЄM" on the other side.
- 10 mg tablets: orange, round, biconvex, film-coated tablets, and debossed with "10" on one side and "LЄM" on the other side.
5. Warnings and Precautions
5.1 CNS Depressant Effects and Daytime Impairment
DAYVIGO is a central nervous system (CNS) depressant that can impair daytime wakefulness even when used as prescribed. CNS depressant effects may persist in some patients for up to several days after discontinuing DAYVIGO. Prescribers should advise patients about the potential for next-day somnolence.
Driving ability was impaired in some subjects taking DAYVIGO 10 mg [see Clinical Studies (14.2)]. The risk of daytime impairment is increased if DAYVIGO is taken with less than a full night of sleep remaining or if a higher than recommended dose is taken [see Dosage and Administration (2.1)]. If DAYVIGO is taken in these circumstances, patients should be cautioned against driving and other activities requiring complete mental alertness.
Co-administration with other CNS depressants (e.g., benzodiazepines, opioids, tricyclic antidepressants, alcohol) increases the risk of CNS depression, which can cause daytime impairment. Dosage adjustments of DAYVIGO and of concomitant CNS depressants may be necessary when administered together because of potentially additive effects. The use of DAYVIGO with other drugs to treat insomnia is not recommended. Patients should be advised not to consume alcohol in combination with DAYVIGO because of additive effects [see Drug Interactions (7.1)].
Because DAYVIGO can cause drowsiness, patients, particularly the elderly, are at a higher risk of falls.
5.2 Sleep Paralysis, Hypnagogic/Hypnopompic Hallucinations, and Cataplexy-like Symptoms
Sleep paralysis, an inability to move or speak for up to several minutes during sleep-wake transitions, and hypnagogic/hypnopompic hallucinations, including vivid and disturbing perceptions, can occur with the use of DAYVIGO. Prescribers should explain the nature of these events to patients when prescribing DAYVIGO.
Symptoms similar to mild cataplexy can occur with DAYVIGO. Such symptoms can include periods of leg weakness lasting from seconds to a few minutes, can occur either at night or during the day, and may not be associated with an identified triggering event (e.g., laughter or surprise).
5.3 Complex Sleep Behaviors
Complex sleep behaviors, including sleep-walking, sleep-driving, and engaging in other activities while not fully awake (e.g., preparing and eating food, making phone calls, having sex), have been reported to occur with the use of hypnotics such as DAYVIGO. These events can occur in hypnotic-naïve as well as in hypnotic-experienced persons. Patients usually do not remember these events. Complex sleep behaviors may occur following the first or any subsequent use of DAYVIGO, with or without the concomitant use of alcohol and other CNS depressants [see Drug Interactions (7.1)]. Discontinue DAYVIGO immediately if a patient experiences a complex sleep behavior.
5.4 Patients with Compromised Respiratory Function
DAYVIGO has been studied in mild to severe Obstructive Sleep Apnea (OSA) and moderate to severe Chronic Obstructive Pulmonary Disease (COPD) in short-term clinical trials. The effect of DAYVIGO on respiratory function should be considered if prescribed to patients with compromised respiratory function [see Use in Specific Populations (8.8)].
5.5 Worsening of Depression/Suicidal Ideation
In clinical studies of DAYVIGO in patients with insomnia, the incidence of suicidal ideation or any suicidal behavior, as assessed by questionnaire, was higher in patients receiving DAYVIGO than in those receiving placebo (0.3% for DAYVIGO 10 mg, 0.4% for DAYVIGO 5 mg, and 0.2% for placebo).
In primarily depressed patients treated with hypnotics, worsening of depression and suicidal thoughts and actions (including completed suicides) have been reported. Suicidal tendencies may be present in such patients and protective measures may be required. Intentional overdose is more common in this group of patients; therefore, the lowest number of tablets that is feasible should be prescribed at any one time.
The emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation.
5.6 Need to Evaluate for Co-morbid Diagnoses
Because sleep disturbances may be the presenting manifestation of a medical and/or psychiatric disorder, treatment of insomnia should be initiated only after careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia or the emergence of new cognitive or behavioral abnormalities may be the result of an unrecognized underlying psychiatric or medical disorder and can emerge during the course of treatment with sleep-promoting drugs such as DAYVIGO.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are discussed in detail in other sections of the labeling:
- CNS Depressant Effects and Daytime Impairment [see Warnings and Precautions (5.1)]
- Sleep Paralysis, Hypnagogic/Hypnopompic Hallucinations, and Cataplexy-like Symptoms [see Warnings and Precautions (5.2)]
- Complex Sleep Behaviors [see Warnings and Precautions (5.3)]
- Patients with Compromised Respiratory Function [see Warnings and Precautions (5.4)]
- Worsening of Depression/Suicidal Ideation [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of DAYVIGO was evaluated in 1418 adult patients with insomnia disorder (age 18 to 88 years) from two controlled efficacy trials (Study 1 and Study 2). Study 1 was a 6-month placebo-controlled trial assessing DAYVIGO 5 or 10 mg once nightly, followed by a 6-month parallel-group extension period in which patients initially treated with DAYVIGO continued on the same dose, and patients who received placebo were re-randomized to receive DAYVIGO 5 or 10 mg once nightly. In Study 1, 434 patients were treated with DAYVIGO for one year. Study 2 was a 30-day placebo- and active-controlled trial assessing DAYVIGO 5 or 10 mg once nightly.
Adverse Reactions Resulting in Discontinuation of Treatment
The frequencies of discontinuation due to adverse reactions in Study 1 (the first 30 days) and Study 2 were 2.6% and 1.4% for patients treated with 10 mg and 5 mg DAYVIGO, respectively, compared to 1.5% for patients in the placebo group. The most common adverse reactions leading to discontinuation of DAYVIGO were somnolence (1.0% for 10 mg, 0.7% for 5 mg, and 0.4% for placebo) and nightmares (0.3% for 10 mg, 0.3% for 5 mg, and 0% for placebo).
The frequencies of discontinuation due to adverse reactions in the 6-month placebo-controlled period of Study 1 were 8.3% and 4.1% for patients treated with DAYVIGO 10 mg and 5 mg, respectively, compared to 3.8% for patients in the placebo group. The most common reasons for discontinuation of DAYVIGO and occurring in more than one patient within a treatment arm were somnolence (2.9% for 10 mg, 1.0% for 5 mg, and 0.6% for placebo), nightmares (1.3% for 10 mg, 0.3% for 5 mg, and 0% for placebo), and palpitations (0.6% for 10 mg, 0% for 5 mg, and 0% for placebo).
Most Common Adverse Reactions
The most common adverse reaction (reported in 5% or more of patients treated with DAYVIGO and at least twice the rate of placebo) in Study 1 (the first 30 days) and Study 2 was somnolence (10% for DAYVIGO 10 mg, 7% for DAYVIGO 5 mg, and 1% for placebo).
Table 1 presents the adverse reactions based on the pooled data from the first 30 days of Study 1 (6-month controlled efficacy trial) and Study 2 (1-month controlled efficacy trial) where the incidence was ≥2% in DAYVIGO-treated patients and greater than in placebo-treated patients.
| Table 1: Adverse Reactions Reported in ≥2% of DAYVIGO-Treated Patients and at a Greater Frequency than Placebo-Treated Patients During the First 30 Days of Study 1 and Study 2 | |||
| DAYVIGO | |||
| Placebo | 5 mg | 10 mg | |
| n=528 | n=580 | n=582 | |
| (%) | (%) | (%) | |
| Somnolence or fatigue* | 1.3 | 6.9 | 9.6 |
| Headache | 3.4 | 5.9 | 4.5 |
| Nightmare or abnormal dreams | 0.9 | 0.9 | 2.2 |
*Combines preferred terms somnolence, lethargy, fatigue, sluggishness
Other Adverse Reactions Observed During Clinical Trials (Studies 1 and 2)
Other adverse reactions of <2% incidence but greater than placebo are shown below. The following list does not include adverse reactions 1) for which a drug cause was remote, 2) that were so general to be uninformative, or 3) that were not considered to have clinically significant implications.
- Sleep paralysis was reported in 1.6% and 1.3% of patients receiving DAYVIGO 10 mg and 5 mg, respectively, compared to no reports for placebo. Hypnagogic hallucinations were reported in 0.7% and 0.1% of patients receiving DAYVIGO 10 mg and 5 mg, respectively, compared to no reports for placebo [see Warnings and Precautions (5.2)].
- Two events of complex sleep behavior were reported, both in patients receiving DAYVIGO 10 mg [see Warnings and Precautions (5.3)].
7. Drug Interactions
7.1 Drugs Having Clinically Important Interactions with DAYVIGO
| Table 2: Clinically Important Drug Interactions with DAYVIGO | |
| Effect of Other Drugs on DAYVIGO | |
| Strong, Moderate, and Weak CYP3A Inhibitors | |
| Clinical Impact: | Concomitant use with a strong, moderate, or weak CYP3A inhibitor increases lemborexant AUC and Cmax which may increase the risk of DAYVIGO adverse reactions [see Clinical Pharmacology (12.3)]. |
| Intervention: | Avoid concomitant use of DAYVIGO with strong or moderate CYP3A inhibitors [see Dosage and Administration (2.2)].
The maximum recommended dose of DAYVIGO with weak CYP3A inhibitors is 5 mg [see Dosage and Administration (2.2)]. |
| Examples: | Strong CYP3A inhibitors: itraconazole, clarithromycin Moderate CYP3A inhibitors: fluconazole, verapamil Weak CYP3A inhibitors: chlorzoxazone, ranitidine |
| Strong and Moderate CYP3A Inducers | |
| Clinical Impact: | Concomitant use with a strong or moderate CYP3A inducer decreases lemborexant exposure, which may reduce DAYVIGO efficacy [see Clinical Pharmacology (12.3)]. |
| Intervention: | Avoid concomitant use of DAYVIGO with strong or moderate CYP3A inducers [see Dosage and Administration (2.2)]. |
| Examples: | Strong CYP3A inducers: rifampin, carbamazepine, St. John’s wort Moderate CYP3A inducers: bosentan, efavirenz, etravirine, modafinil |
| Alcohol | |
| Clinical Impact: | Concomitant use of alcohol increases lemborexant Cmax and AUC. Co-administration of DAYVIGO with alcohol produced a numerically greater negative impact on postural stability and memory as compared with alcohol alone when assessed near the tmax of DAYVIGO (2 hours post-dose) [see Clinical Pharmacology (12.2)]. |
| Intervention: | Avoid alcohol consumption with DAYVIGO [see Warnings and Precautions (5.1)]. |
| Effect of DAYVIGO on Other Drugs | |
| CYP2B6 Substrates | |
| Clinical Impact: | Concomitant use of DAYVIGO decreases the AUC of drugs that are CYP2B6 substrates, which may result in reduced efficacy for these concomitant medications [see Clinical Pharmacology (12.3)]. |
| Intervention: | Patients receiving DAYVIGO and CYP2B6 substrates concurrently should be monitored for adequate clinical response. Increasing the doses of CYP2B6 substrates may be considered as needed. |
| Examples: | Bupropion, methadone |
8. Use In Specific Populations
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women who are exposed to DAYVIGO during pregnancy. Healthcare providers are encouraged to register patients in the DAYVIGO pregnancy registry by calling 1-888-274-2378.
Risk Summary
There are no available data on DAYVIGO use in pregnant women to evaluate for drug-associated risks of major birth defects, miscarriage or adverse maternal or fetal outcomes.
In animal reproduction studies, oral administration of lemborexant to pregnant rats and rabbits during the period of organogenesis caused toxicities only at high multiples of the human exposure at the maximum recommended human dose (MRHD) based on AUC. The no observed adverse effect levels (NOAEL) are approximately >100 and 23 times the MRHD based on AUC in rats and rabbits, respectively. Similarly, oral administration of lemborexant to pregnant and lactating rats caused toxicities only at high multiples of the human exposure at the MRHD based on AUC. The NOAEL is 93 times the MRHD based on AUC (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Lemborexant was administered orally to pregnant rats during the period of organogenesis in 2 studies at doses of 60, 200, and 600 mg/kg/day or 20, 60, and 200 mg/kg/day, which are approximately 6 to >300 times the MRHD based on AUC. Lemborexant caused maternal toxicity, manifested by decreased body weight and food consumption, decreased mean fetal body weight, an increased number of dead fetuses, and skeletal, external and visceral malformations (omphalocele, cleft palate, and membranous ventricular septal defect) at >300 times the MRHD based on AUC. The NOAEL of 200 mg/kg/day is approximately 143 times the MRHD based on AUC.
Lemborexant was administered orally to pregnant rabbits during the period of organogenesis at doses of 10, 30, and 100 mg/kg/day, which are approximately 7 to 139 times the MRHD based on AUC. Lemborexant caused maternal toxicity that consisted of decreased body weight and food consumption and a higher incidence of skeletal variations (presence of cervical ribs and supernumerary lung lobes) at approximately 139 times the MRHD based on AUC. The NOAEL of 30 mg/kg/day is approximately 23 times the MRHD based on AUC.
Lemborexant was administered orally to pregnant rats during pregnancy and lactation at doses of 30, 100, and 300 mg/kg/day, which are approximately 15 to 206 times the MRHD based on AUC. Lemborexant caused maternal toxicity that consisted of decreased body weight and food consumption and toxicity to offspring consisting of decreased pup body weights, decreased femur length, and decreased acoustic startle responses at 206 times the MRHD based on AUC. The NOAEL of 100 mg/kg/day is approximately 93 times the MRHD based on AUC.
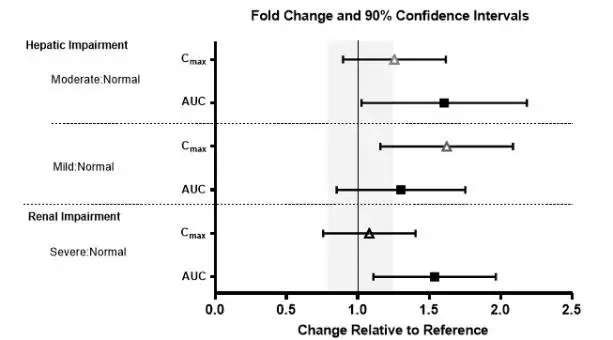
8.7 Hepatic Impairment
DAYVIGO has not been studied in patients with severe hepatic impairment. Use in this population is not recommended [see Dosage and Administration (2.3), Clinical Pharmacology (12.3)].
DAYVIGO exposure (AUC and Cmax) and terminal half-life were increased in patients with moderate hepatic impairment (Child-Pugh class B). Dosage adjustment is recommended in patients with moderate hepatic impairment (Child-Pugh class B) [see Dosage and Administration (2.3), Clinical Pharmacology (12.3)].
DAYVIGO exposure (AUC) was increased in patients with mild hepatic impairment (Child-Pugh class A), but the terminal half-life was not changed. Patients with mild hepatic impairment may experience an increased risk of somnolence [see Clinical Pharmacology (12.3)].
8.8 Patients with Compromised Respiratory Function
Obstructive Sleep Apnea (OSA)
The respiratory depressant effect of DAYVIGO was evaluated after 8 consecutive nights of treatment with DAYVIGO 10 mg in a randomized, placebo-controlled, two-period crossover study in 37 patients with mild OSA (apnea-hypopnea index < 15 events per hour of sleep). Following once daily dosing of 10 mg, the mean treatment difference (DAYVIGO – placebo) on Day 8 for the apnea-hypopnea index was -0.06 (95% CI: -1.95 to 1.83).
DAYVIGO was also evaluated after 8 consecutive nights of treatment with DAYVIGO 10 mg in a randomized, placebo-controlled, two-period crossover study in 33 patients with moderate to severe OSA (apnea-hypopnea index ≥ 15 events per hour of sleep). Following once daily dosing of 10 mg, the mean treatment difference (DAYVIGO – placebo) on Day 8 for the apnea-hypopnea index was -0.80 (95% CI: -4.88 to 3.29).
Due to study limitations, including the short duration of the study, clinically meaningful respiratory effects of DAYVIGO in OSA cannot be excluded, including for long-term treatment [see Warnings and Precautions (5.4)].
Chronic Obstructive Pulmonary Disease (COPD)
The respiratory depressant effect of DAYVIGO was evaluated after 8 consecutive nights of treatment with DAYVIGO 10 mg in a randomized, placebo-controlled, two-period crossover study in 30 patients with moderate to severe COPD (Forced expiratory volume in the first second (FEV1)/Forced vital capacity (FVC) ratio ≤ 70% and 30% ≤ FEV1 < 80% of predicted). Following once-daily dosing of 10 mg, the mean treatment difference (DAYVIGO – placebo) on Day 8 for the mean peripheral capillary oxygen saturation during sleep was 0.47 (95% CI: 0.07 to 0.87).
DAYVIGO has not been studied in COPD patients with a FEV1 < 30% of predicted.
Clinically meaningful respiratory effects of DAYVIGO in patients with compromised respiratory function cannot be excluded [see Warnings and Precautions (5.4)].
12. Dayvigo - Clinical Pharmacology
12.2 Pharmacodynamics
Lemborexant binds to orexin receptors OX1R and OX2R and acts as a competitive antagonist (IC50 values of 6.1 nM and 2.6 nM, respectively). A major metabolite of lemborexant, M10, binds with comparable affinity as the parent drug to orexin receptors OX1R and OX2R (IC50 values of 4.2 nM and 2.9 nM), respectively.
Cardiac Electrophysiology
In a concentration-QTcF analysis using the data from two randomized, double-blind, placebo-controlled, multiple ascending dose studies in healthy subjects, lemborexant does not prolong the QTcF interval to any clinically relevant extent at a dose 5 times the maximum recommended dose.
Drug Interactions
Lemborexant co-administered with alcohol produced a numerically greater negative impact on postural stability and memory as compared with alcohol alone at approximately 2 hours post-dose [see Drug Interactions 7.1].
12.3 Pharmacokinetics
Following single doses of lemborexant 2.5 to 75 mg, geometric mean Cmax and AUC0-24h increased slightly less than in proportion to dose. The extent of accumulation of lemborexant at steady-state is 1.5- to 3-fold across this dose range.
Absorption
The time to peak concentration (tmax) of lemborexant is approximately 1 to 3 hours.
Effect of Food
Lemborexant Cmax decreased by 23%, AUC0-inf increased by 18%, and tmax was delayed by 2 hours following administration of a high-fat and high-calorie meal (containing approximately 150, 250, and 500 to 600 calories from protein, carbohydrate, and fat, respectively).
Distribution
The volume of distribution of lemborexant is 1970 L. Plasma protein binding of lemborexant is approximately 88% in vitro and 94% in clinical samples. The blood to plasma concentration ratio of lemborexant is 0.65.
Elimination
Metabolism
Lemborexant is primarily metabolized by CYP3A4, and to a lesser extent by CYP3A5. The major circulating metabolite is M10.
Excretion
Following administration of an oral dose, 57.4% of the dose was recovered in the feces and 29.1% in the urine (<1% as unchanged). The effective half-life for lemborexant 5 mg and 10 mg is 17 and 19 hours, respectively.
Specific Populations
No clinically significant differences in the pharmacokinetics of lemborexant were observed based on age, sex, race/ethnicity, or body mass index. No studies have been conducted to investigate the pharmacokinetics of lemborexant in pediatric patients. Exposures of lemborexant in patients with hepatic and renal impairment are summarized in Figure 1.
Figure 1. Effects of Hepatic and Renal Impairment on Lemborexant Pharmacokinetics
Drug Interaction Studies
The effects of other drugs on the exposures of lemborexant are summarized in Figure 2. The effects of lemborexant on the exposures of other drugs are summarized in Figure 3. Based on these results, drug interactions between lemborexant and strong CYP3A inducers, strong CYP3A inhibitors, moderate CYP3A inhibitors, and CYP2B6 substrates are clinically significant [see Dosage and Administration (2.2), Drug Interactions (7.1)].
Physiologically-based pharmacokinetic (PBPK) modeling predicted that concomitant use of weak CYP3A inhibitors increased lemborexant exposure by less than 2-fold [see Dosage and Administration (2.2), Drug Interactions (7.1)], and that lemborexant is expected to have minimal effect on the pharmacokinetics of CYP2C8, CYP2C9, or CYP2C19 substrates.
Figure 2. Effects of Co-administered Drugs on the Pharmacokinetics of Lemborexant 10 mg
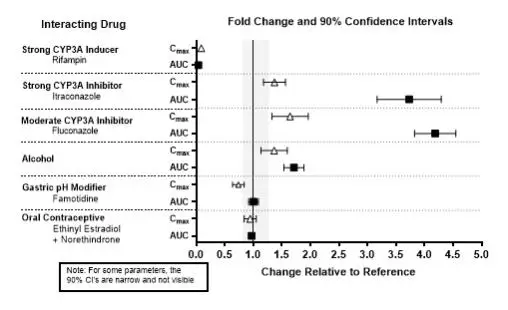
Figure 3. Effects of Lemborexant 10 mg on the Pharmacokinetics of Co-Administered Drugs
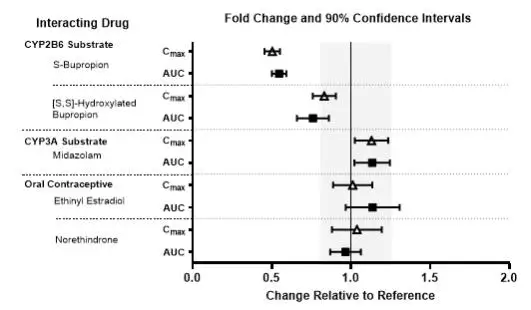
In Vitro Studies
In vitro metabolism studies demonstrated that lemborexant and M10 have the potential to induce CYP3A and a weak potential to inhibit CYP3A and induce CYP2B6. Lemborexant and M10 do not inhibit other CYP isoforms (CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP2E1) or transporters (P-gp, BCRP, BSEP, OAT1, OAT3, OATP1B1, OATP1B3, OCT1, OCT2, MATE1, and MATE2-K). Lemborexant and M10 do not induce CYP2C8, CYP2C9, and CYP2C19 at clinically relevant concentrations.
Lemborexant is a potential poor substrate of P-gp, but M10 is a substrate of P-gp. Lemborexant and M10 are not substrates of BCRP, OATP1B1, or OATP1B3.
14. Clinical Studies
14.1 Controlled Clinical Studies
DAYVIGO was evaluated in two clinical trials in patients with insomnia characterized by difficulties with sleep onset and/or sleep maintenance (Study 1, NCT02952820 and Study 2, NCT02783729).
Study 1 was a 6-month, randomized, double-blind, placebo-controlled, multi-center trial in adult patients age 18 or older who met DSM-5 criteria for insomnia disorder. Patients were randomized to placebo (n=325), DAYVIGO 5 mg (n=323), or DAYVIGO 10 mg (n=323) once nightly. The primary efficacy endpoint was the mean change from baseline to end of treatment at 6 months for log-transformed patient-reported (subjective) sleep onset latency (sSOL), defined as the estimated minutes from the time that the patient attempted to sleep until sleep onset. Pre-specified secondary efficacy endpoints for sleep maintenance were change from baseline to end of treatment at 6 months for patient-reported sleep efficiency (sSEF) and wake after sleep onset (sWASO). sSEF is defined as the proportion of time spent asleep per time in bed. sWASO is defined as the minutes of wake from the onset of sleep until wake time. The primary and pre-specified secondary efficacy endpoints were measured by sleep diary.
The demographic characteristics of patients in Study 1 were similar across the treatment arms. Patients had a median age of 55 years (range 18 to 88) and were 68% female, 72% White, 8% Black or African American, 17% Japanese, and 3.5% other; 28% were elderly (≥65 years).
Examination of subgroups by age, race, and sex did not suggest differences in response to DAYVIGO. In Study 1, DAYVIGO 5 mg and 10 mg demonstrated statistically significant superiority on the primary efficacy measure, sSOL, compared to placebo (Table 3). DAYVIGO 5 mg and 10 mg also showed statistically significant superiority in sSEF and sWASO.
Table 3: Primary and Secondary Efficacy Results for Change from Baseline in Sleep Onset and Sleep Maintenance at 6 Months in Patients with Insomnia (Study 1)
| Endpoint | Treatment Group | Number of Patients
ITT | Baseline Meana
(SD) | Month 6
LS Meana (SE) | Treatment Effect
(95% CI) |
| Sleep Onset
sSOL (minutes) | DAYVIGO 5 mg* | 316 | 43.0 (31.5) | 20.0 (1.1) | 0.7 (0.6, 0.8) |
| DAYVIGO 10 mg* | 315 | 45.0 (33.4) | 19.2 (1.1) | 0.7 (0.6, 0.8) | |
| Placebo | 318 | 45.0 (31.8) | 27.3 (1.4) | (ratio vs placebo)b | |
| Sleep Maintenance
sSEF (%) | DAYVIGO 5 mg* | 316 | 63.1 (18.2) | 75.9 (0.9) | 4.5 (2.2, 6.9) |
| DAYVIGO 10 mg* | 315 | 62.0 (17.2) | 75.9 (0.9) | 4.7 (2.4, 7.0) | |
| Placebo | 318 | 61.3 (17.8) | 71.4 (0.8) | (%)c | |
| Sleep Maintenance
sWASO (minutes) | DAYVIGO 5 mg* | 316 | 132.8 (82.5) | 87.9 (3.7) | -17.5 (-27.3, -7.6) |
| DAYVIGO 10 mg* | 315 | 136.8 (87.4) | 92.7 (3.7) | -12.7 (-22.4, -3.0) | |
| Placebo | 318 | 132.5 (80.2) | 105.3 (3.6) | (minutes)c | |
| ITT (intention to treat); sSOL (subjective sleep onset latency); SD (standard deviation); LS (least squares); SE (standard error); CI (unadjusted confidence interval); sSEF (subjective sleep efficiency); sWASO (subjective wake after sleep onset) a For the sleep onset sSOL endpoint, the mean refers to geometric mean, which was used due to the approximately log normal distribution of the outcomes; SD for the geometric mean is calculated as GM*SD (log transformed sSOL); SE for the least squares geometric mean is calculated in the same way as the SD. b For the sleep onset sSOL endpoint, treatment effect refers to the ratio of [Month 6 sSOL / Baseline sSOL] for DAYVIGO versus placebo, such that a smaller ratio corresponds to a greater improvement. c Treatment effect refers to the treatment difference between DAYVIGO versus placebo, such that a larger value for sSEF and smaller value for sWASO correspond to greater improvement. * Doses that were statistically significantly superior (p<0.05) to placebo after multiplicity adjustment. |
|||||
Study 2 was a 1-month, randomized, double-blind, placebo- and active-controlled, multi-center, parallel-group clinical trial in adult female patients age 55 and older and male patients 65 years and older who met DSM-5 criteria for insomnia disorder. Patients were randomized to placebo (n=208), DAYVIGO 5 mg (n=266) or 10 mg (n=269), or active comparator (n=263) once nightly.
The primary efficacy endpoint was the mean change in log-transformed latency to persistent sleep (LPS) from baseline to end of treatment (Days 29/30), as measured by overnight polysomnography (PSG) monitoring. LPS was defined as the number of minutes from lights off to the first 10 consecutive minutes of non-wakefulness. The pre-specified secondary efficacy endpoints in Study 2 were the mean change from baseline to end of treatment (Days 29/30) in sleep efficiency (SEF) and wake after sleep onset (WASO) measured by PSG.
The demographic and baseline characteristics of patients in Study 2 were similar across the treatment arms. Patients had a median age of 63 years (range 55 to 88) and were 86% female, 72% White, 25% Black or African American, and 2% other; 45% were elderly (≥65 years).
In Study 2, DAYVIGO 5 mg and 10 mg demonstrated statistically significant superiority on the primary efficacy measure, LPS, compared to placebo (Table 4). DAYVIGO 5 mg and 10 mg demonstrated statistically significant improvement in SEF and WASO compared to placebo.
Table 4: Primary and Secondary Efficacy Results for Change from Baseline in Sleep Onset and Sleep Maintenance at 1 Month in Patients with Insomnia (Study 2)
| Endpoint | Treatment Group | Number of Patients
ITT | Baseline Meana
(SD) | Day 29/30
LS Meana (SE) | Treatment Effect
(95% CI) |
| Sleep Onset
LPS (minutes) | DAYVIGO 5 mg* | 266 | 33.0 (27.2) | 15.5 (0.8) | 0.8 (0.7, 0.9) |
| DAYVIGO 10 mg* | 269 | 33.3 (27.2) | 14.5 (0.7) | 0.7 (0.6, 0.8) | |
| Placebo | 208 | 33.6 (25.9) | 20.0 (1.1) | (ratio vs. placebo)b | |
| Sleep Maintenance
SEF (%) | DAYVIGO 5 mg* | 266 | 68.4 (11.3) | 80.7 (0.5) | 7.1 (5.6, 8.5) |
| DAYVIGO 10 mg* | 269 | 67.8 (10.8) | 82.7 (0.5) | 8.0 (6.6, 9.5) | |
| Placebo | 208 | 68.9 (9.6) | 74.6 (0.6) | (%)c | |
| Sleep Maintenance
WASO (minutes) | DAYVIGO 5 mg* | 266 | 113.4 (39.0) | 68.3 (2.2) | -24.0 (-30.0, -18.0) |
| DAYVIGO 10 mg* | 269 | 114.8 (40.0) | 66.9 (2.2) | -25.3 (-31.4, -19.3) | |
| Placebo | 208 | 111.7 (37.2) | 92.2 (2.5) | (minutes)c | |
| ITT (intention to treat); LPS (latency to persistent sleep); SD (standard deviation); LS (least squares); SE (standard error); CI (unadjusted confidence interval); SEF (sleep efficiency); WASO (wake after sleep onset) a For the sleep onset LPS endpoint, the mean refers to geometric mean, which was used due to the approximately log normal distribution of the outcomes; SD for the geometric mean is calculated as GM*SD (log transformed LPS); SE for the least squares geometric mean is calculated in the same way as the SD. b For the LPS endpoint, treatment effect refers to the ratio of [Day 29/30 LPS / Baseline LPS] for DAYVIGO versus placebo, such that a smaller ratio corresponds to a greater improvement. c Treatment effect refers to the treatment difference between DAYVIGO versus placebo, such that a larger value for SEF and smaller value for WASO correspond to greater improvement. * Doses that were statistically significantly superior (p<0.05) to placebo after multiplicity adjustment. |
|||||
The effects of DAYVIGO at the beginning of treatment were generally consistent with later timepoints.
14.2 Special Safety Studies
Middle of the Night Safety
The effect of DAYVIGO on middle of the night safety was evaluated in a randomized, placebo- and active-controlled trial in healthy female subjects ≥55 years or male subjects ≥65 years. Postural stability, the ability to awaken in response to a sound stimulus, and attention and memory were assessed following a scheduled awakening 4 hours after the start of the 8-hour time in bed. Postural stability was measured by assessing body sway using an ataxia meter. Nighttime dosing of DAYVIGO 5 mg and 10 mg resulted in impairment of balance (measured by body sway area) at 4 hours as compared to placebo.
The ability to awaken to sound in the middle of the night was assessed using an audiometer that delivered 1000 Hz tones up to 105 dB. There were no meaningful differences between DAYVIGO (5 mg or 10 mg) and placebo on ability to awaken to sound.
A computerized performance assessment battery was administered to assess attention and memory after middle of the night awakening (4 hours postdose) in subjects receiving DAYVIGO 5 mg or 10 mg. DAYVIGO was associated with dose-dependent worsening on measures of attention and memory as compared to placebo.
Patients should be cautioned about the potential for middle of the night postural instability, as well as attention and memory impairment.
Effects on Next-day Postural Stability and Memory
The effects of DAYVIGO on next day postural stability and memory were evaluated in two randomized, placebo- and active-controlled trials in healthy subjects and insomnia patients age 55 and older.
There were no meaningful differences between DAYVIGO (5 mg or 10 mg) and placebo on next-day postural stability or memory compared to placebo.
Effects on Driving
A randomized, double-blind, placebo- and active-controlled, four-period crossover study evaluated the effects of nighttime administration of DAYVIGO on next-morning driving performance approximately 9 hours after dosing in 24 healthy elderly subjects (≥65 years, median age 67 years; 14 men, 10 women) and 24 adult subjects (median age 49 years; 12 men, 12 women). The primary driving performance outcome measure was change in Standard Deviation of Lateral Position (SDLP). Testing was conducted after one night (a single dose) and after eight consecutive nights of treatment with DAYVIGO. Although DAYVIGO at doses of 5 mg and 10 mg did not cause statistically significant impairment in next-morning driving performance in adult or elderly subjects (compared with placebo), driving ability was impaired in some subjects taking 10 mg DAYVIGO.
Patients using the 10 mg dose should be cautioned about the potential for next-morning driving impairment because there is individual variation in sensitivity to DAYVIGO.
Rebound Insomnia
Rebound insomnia was assessed by comparing sleep diary-recorded sSOL and sWASO from the screening period to the two weeks following treatment discontinuation in both Studies 1 and 2. Analyses of group means and the proportion of patients with rebound insomnia suggest that DAYVIGO was not associated with rebound insomnia following treatment discontinuation.
Withdrawal Effects
In 12-month and 1-month controlled safety and efficacy trials (Studies 1 and 2, respectively), withdrawal effects were assessed by the Tyrer Benzodiazepine Withdrawal Symptom Questionnaire following discontinuation from study drug in patients who received DAYVIGO 5 mg or 10 mg. There was no evidence of withdrawal effects following DAYVIGO discontinuation at either dose.
Medication Guide
| MEDICATION GUIDE
DAYVIGO® (daye-vi'-goe) (lemborexant) tablets, for oral use, CIV |
| What is the most important information I should know about DAYVIGO?
DAYVIGO may cause serious side effects including:
|
What is DAYVIGO?
|
| DAYVIGO is a federally controlled substance (CIV) because it can be abused or cause dependence. Keep DAYVIGO in a safe place to prevent misuse and abuse. Selling or giving away DAYVIGO may harm others and is against the law. Tell your doctor if you have ever abused or have been dependent on alcohol, prescription medicines or street drugs. |
| Who should not take DAYVIGO?
Do not take DAYVIGO if you fall asleep often at unexpected times (narcolepsy). |
Before taking DAYVIGO, tell your healthcare provider about all of your medical conditions, including if you:
|
How should I take DAYVIGO?
|
What should I avoid while taking DAYVIGO?
|
| What are the possible side effects of DAYVIGO? See “What is the most important information I should know about DAYVIGO?”
DAYVIGO may cause serious side effects, including:
These are not all of the possible side effects of DAYVIGO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
How should I store DAYVIGO?
|
| General information about the safe and effective use of DAYVIGO.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use DAYVIGO for a condition for which it was not prescribed. Do not give DAYVIGO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about DAYVIGO that is written for healthcare professionals. |
| What are the ingredients in DAYVIGO?
Active ingredient: lemborexant Inactive ingredients: hydroxypropyl cellulose, lactose monohydrate, low-substituted hydroxypropyl cellulose, and magnesium stearate. The tablet film coating contains: hypromellose 2910, polyethylene glycol 8000, talc, titanium dioxide, and either ferric oxide yellow for the 5 mg tablet; or both ferric oxide yellow and ferric oxide red for the 10 mg tablet. |
| Distributed by: Eisai Inc. Nutley, NJ 07110 DAYVIGO® is a registered trademark of Eisai R&D Management Co., Ltd. and is licensed to Eisai Inc. © 2019-2023 Eisai Inc. For more information, go to www.DAYVIGO.com or call 1-888-274-2378 |
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 04/2023
| DAYVIGO
lemborexant tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| DAYVIGO
lemborexant tablet, film coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| DAYVIGO
lemborexant tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Eisai Inc. (189246791) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent Pharma Solutions, LLC | 014167995 | analysis(62856-405, 62856-410, 62856-455) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eisai Co., Ltd. | 695319983 | analysis(62856-405, 62856-410, 62856-455) , api manufacture(62856-405, 62856-410, 62856-455) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eisai Manufacturing Ltd | 219516916 | analysis(62856-405, 62856-410, 62856-455) , manufacture(62856-405, 62856-410, 62856-455) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharma Packaging Solutions, LLC dba Tjoapack, LLC | 928861723 | label(62856-405, 62856-410, 62856-455) , pack(62856-405, 62856-410, 62856-455) | |




![The structural formula for DAYVIGO contains lemborexant, an orexin receptor antagonist. The chemical name of lemborexant is (1R,2S)-2-{[(2,4-dimethylpyrimidin-5-yl)oxy]methyl}-2-(3-fluorophenyl)-N-(5-fluoropyridin-2-yl) cyclopropanecarboxamide. The molecular formula is C22H20F2N4O2. The molecular weight is 410.42.](https://cdn.themeditary.com/images/2023/08/31/dayvigo-01.webp)