Drug Detail:Enstilar (Calcipotriene and betamethasone dipropionate)
Drug Class: Topical antipsoriatics
Highlights of Prescribing Information
ENSTILAR® (calcipotriene and betamethasone dipropionate) foam, for topical use
Initial U.S. Approval: 2006
Indications and Usage for Enstilar
Enstilar Foam is a combination of calcipotriene, a vitamin D analog, and betamethasone dipropionate, a corticosteroid, indicated for the topical treatment of plaque psoriasis in patients 12 years and older. (1)
Enstilar Dosage and Administration
- Shake before use. Apply Enstilar Foam to affected area(s) once daily for up to 4 weeks. Discontinue therapy when control is achieved. Do not use more than 60 grams every 4 days. (2)
- Do not use with occlusive dressings unless directed by a healthcare provider. (2)
- Avoid use on the face, groin, or axillae, or if skin atrophy is present at the treatment site. (2)
- Not for oral, ophthalmic, or intravaginal use. (2)
Dosage Forms and Strengths
Foam: 0.005%/0.064% - Each gram of Enstilar Foam contains 50 mcg of calcipotriene and 0.643 mg of betamethasone dipropionate. (3)
Contraindications
None. (4)
Warnings and Precautions
- Flammability: The propellants in Enstilar Foam are flammable. Instruct the patient to avoid fire, flame, and smoking during and immediately following application. (5.1)
- Hypercalcemia and Hypercalciuria: Hypercalcemia and hypercalciuria have been observed with use of Enstilar Foam. If hypercalcemia or hypercalciuria develop, discontinue treatment until parameters of calcium metabolism have normalized. (5.2)
- Effects on Endocrine System: Topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency during and after withdrawal of treatment. Risk factors include the use of high-potency topical corticosteroids, use over a large surface area or on areas under occlusion, prolonged use, altered skin barrier, liver failure, and use in pediatric patients. Modify use should HPA axis suppression develop. (5.3, 8.4)
- Ophthalmic Adverse Reactions: Topical corticosteroid products may increase the risk of cataracts and glaucoma. If visual symptoms occur, consider referral to an ophthalmologist. (5.5)
Adverse Reactions/Side Effects
Adverse reactions reported in < 1% of subjects included application site irritation, application site pruritus, folliculitis, skin hypopigmentation, hypercalcemia, urticaria, and exacerbation of psoriasis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact LEO Pharma Inc. at 1-877-494-4536 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2022
Full Prescribing Information
1. Indications and Usage for Enstilar
Enstilar® (calcipotriene and betamethasone dipropionate) Foam is indicated for the topical treatment of plaque psoriasis in patients 12 years and older.
2. Enstilar Dosage and Administration
Shake can prior to using Enstilar Foam. Apply Enstilar Foam to affected areas once daily for up to 4 weeks. The maximum dose should not exceed 60 grams every 4 days. Rub in Enstilar Foam gently. Wash hands after applying the product. Discontinue Enstilar Foam when control is achieved.
Enstilar Foam should not be used:
- with occlusive dressings unless directed by a healthcare provider.
- on the face, groin, or axillae, or if skin atrophy is present at the treatment site.
Enstilar Foam is not for oral, ophthalmic, or intravaginal use.
3. Dosage Forms and Strengths
Enstilar Foam: 0.005%/0.064% - each gram contains 50 mcg calcipotriene and 0.643 mg of betamethasone dipropionate in a white to off-white opalescent liquid in a pressurized aluminum spray can with a continuous valve and actuator. At administration the product is a white to off-white foam after evaporation of the propellants.
5. Warnings and Precautions
5.1 Flammability
The propellants in Enstilar Foam are flammable. Instruct the patient to avoid fire, flame, and smoking during and immediately following application.
5.2 Hypercalcemia and Hypercalciuria
Hypercalcemia and hypercalciuria have been observed with use of Enstilar Foam. If hypercalcemia or hypercalciuria develop, discontinue treatment until parameters of calcium metabolism have normalized. The incidence of hypercalcemia and hypercalciuria following Enstilar Foam treatment of more than 56 weeks has not been evaluated [see Clinical Pharmacology (12.2)].
5.4 Allergic Contact Dermatitis
Allergic contact dermatitis has been observed with topical calcipotriene and topical corticosteroids. Allergic contact dermatitis to a topical corticosteroid is usually diagnosed by observing a failure to heal rather than a clinical exacerbation. Corroborate such an observation with appropriate diagnostic patch testing.
5.5 Ophthalmic Adverse Reactions
Use of topical corticosteroids, including Enstilar® Foam, may increase the risk of posterior subcapsular cataracts and glaucoma. Cataracts and glaucoma have been reported with the postmarketing use of topical corticosteroid products. Avoid contact with Enstilar Foam with eyes. Enstilar Foam may cause eye irritation. Advise patients to report any visual symptoms and consider referral to an ophthalmologist for evaluation.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.2 Postmarketing Experience
Because adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Postmarketing reports for local adverse reactions to Enstilar Foam included application site burning.
Postmarketing reports for local adverse reactions to topical corticosteroids included atrophy, striae, telangiectasia, dryness, perioral dermatitis, secondary infection, and miliaria.
Ophthalmic adverse reactions of cataracts, glaucoma, and increased intraocular pressure have been reported with the use of topical corticosteroids, including topical betamethasone products.
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of Enstilar Foam for the treatment of mild to severe plaque psoriasis have been established in pediatric patients age 12 to 17 years. The use of Enstilar Foam for this indication is supported by evidence from adequate and well-controlled trials in adults and from one uncontrolled trial in 106 adolescents age 12 to 17 years with psoriasis of the body and scalp. Calcium metabolism was evaluated in all pediatric subjects and no cases of hypercalcemia or clinically relevant changes in urinary calcium were reported. Hypothalamic pituitary adrenal (HPA) axis suppression was evaluated in a subset of 33 pediatric subjects with moderate plaque psoriasis of the body and scalp (mean body surface area involvement of 16% and mean scalp area involvement of 56%). After 4 weeks of once daily treatment with a mean weekly dose of 47 grams, HPA axis suppression was observed in 3 of 33 subjects (9%) [see Warnings and Precautions (5.2), Adverse Reactions (6.1) and Clinical Pharmacology (12.2)].
Because of a higher ratio of skin surface area to body mass, children under the age of 12 years are at particular risk of systemic adverse effects when they are treated with topical corticosteroids. Pediatric patients are, therefore, also at greater risk of HPA axis suppression and adrenal insufficiency with the use of topical corticosteroids including Enstilar Foam [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.2)].
Cushing's syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in pediatric patients treated with topical corticosteroids.
Local adverse reactions including striae have been reported with use of topical corticosteroids in pediatric patients.
The safety and effectiveness of Enstilar Foam in pediatric patients less than 12 years of age have not been established.
8.5 Geriatric Use
Of the total number of subjects in the controlled clinical studies of Enstilar Foam, 97 subjects were 65 years and over, and 21 were 75 and over.
No overall differences in safety or effectiveness of Enstilar Foam were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
11. Enstilar Description
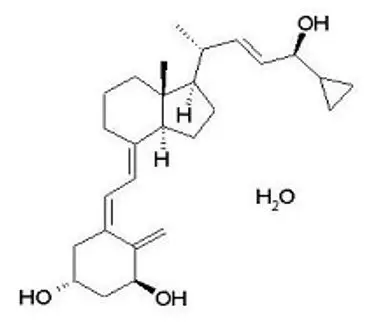
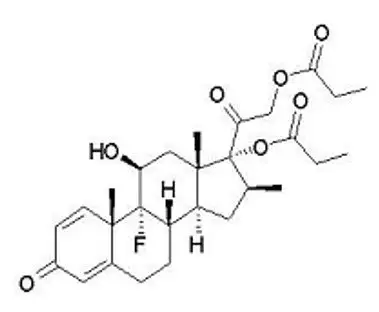
Enstilar Foam contains calcipotriene hydrate and betamethasone dipropionate. It is for topical use only.
12. Enstilar - Clinical Pharmacology
12.1 Mechanism of Action
Enstilar Foam combines the pharmacological effects of calcipotriene hydrate as a synthetic vitamin D3 analog and betamethasone dipropionate as a synthetic corticosteroid. However, while their pharmacologic and clinical effects are known, the exact mechanisms of their actions in the treatment of plaque psoriasis are unknown.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
When calcipotriene was applied topically to mice for up to 24 months at dosages of 3, 10, and 30 mcg/kg/day (9, 30, and 90 mcg/m2/day, respectively), no significant changes in tumor incidence were observed when compared to control.
A 104-week oral carcinogenicity study was conducted with calcipotriene in male and female rats at doses of 1, 5, and 15 mcg/kg/day (6, 30, and 90 mcg/m2/day, respectively). Beginning week 71, the dosage for high-dose animals of both genders was reduced to 10 mcg/kg/day (60 mcg/m2/day). A treatment-related increase in benign C-cell adenomas was observed in the thyroid of females that received 15 mcg/kg/day. A treatment-related increase in benign pheochromocytomas was observed in the adrenal glands of males that received 15 mcg/kg/day. No other statistically significant differences in tumor incidence were observed when compared to control. The relevance of these findings to patients is unknown.
When betamethasone dipropionate was applied topically to CD-1 mice for up to 24 months at dosages approximating 1.3, 4.2, and 8.5 mcg/kg/day in females, and 1.3, 4.2, and 12.9 mcg/kg/day in males (up to 26 mcg/m2/day and 39 mcg/m2/day, in females and males, respectively), no significant changes in tumor incidence were observed when compared to control.
When betamethasone dipropionate was administered via oral gavage to male and female Sprague Dawley rats for up to 24 months at dosages of 20, 60, and 200 mcg/kg/day (120, 360, and 1200 mcg/m2/day, respectively), no significant changes in tumor incidence were observed when compared to control.
Calcipotriene did not elicit any genotoxic effects in the Ames mutagenicity assay, the mouse lymphoma TK locus assay, the human lymphocyte chromosome aberration test, or the mouse micronucleus test. Betamethasone dipropionate did not elicit any genotoxic effects in the Ames mutagenicity assay, the mouse lymphoma TK locus assay, or in the rat micronucleus test.
Studies in rats with oral doses of up to 54 mcg/kg/day (324 mcg/m2/day) of calcipotriene indicated no impairment of fertility or general reproductive performance. Studies in male rats at oral doses of up to 200 mcg/kg/day (1200 mcg/m2/day), and in female rats at oral doses of up to 1000 mcg/kg/day (6000 mcg/m2/day), of betamethasone dipropionate indicated no impairment of fertility.
14. Clinical Studies
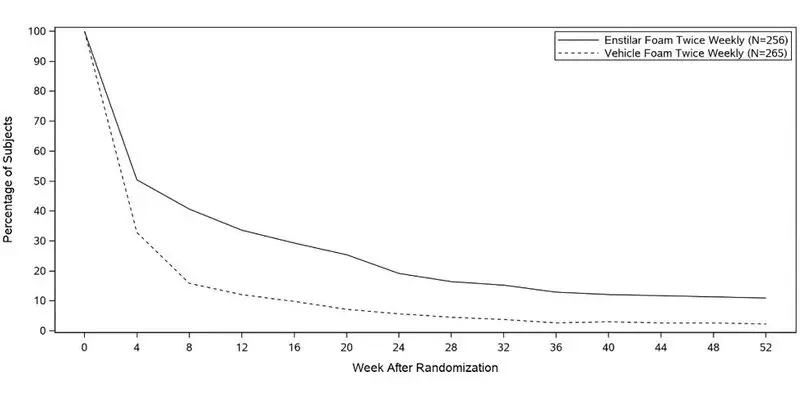
Two multicenter, randomized, double-blind trials were conducted in adult subjects with plaque psoriasis.
- In Trial One, 302 subjects were randomized to 1 of 3 treatment groups: Enstilar® Foam, betamethasone dipropionate in the same vehicle, or calcipotriene in the same vehicle.
- In Trial Two, 426 subjects were randomized to one of two treatment groups: Enstilar Foam or the vehicle alone. Baseline disease severity was graded using a 5-point Investigator's Global Assessment (IGA). At baseline subjects scored "Mild", "Moderate", or "Severe". The majority of subjects in both trials (76% and 75%) had disease of "Moderate" severity at baseline, 14% and 15% of subjects had disease of "Mild" severity at baseline and 10% of subjects had "Severe" disease at baseline in both trials. The extent of disease involvement assessed by mean body surface area was 7.1% (range 2 to 28%) and 7.5% (range 2 to 30%). In both trials, subjects were treated once daily for up to 4 weeks.
Efficacy was assessed with treatment success defined as the proportion of subjects at Week 4 who were "Clear" or "Almost Clear" according to the IGA. Subjects with "Mild" disease at baseline were required to be "Clear" to be considered a treatment success. Table 1 presents the efficacy results for these trials.
| Enstilar Foam | Betamethasone dipropionate in vehicle | Calcipotriene in vehicle | Vehicle | |
|---|---|---|---|---|
|
||||
| Trial One
Week 4 | (N=100) 45.0% | (N=101) 30.7% | (N=101) 14.9% | - - |
| Trial Two
Week 4 | (N=323) 53.3% | - - | - - | (N=103) 4.8% |
16. How is Enstilar supplied
16.1 How Supplied
Enstilar (calcipotriene and betamethasone dipropionate) Foam, 0.005%/0.064% is a white to off-white opalescent liquid in a pressurized aluminum spray can with a continuous valve and actuator. At administration, the product is a white to off-white foam after evaporation of the propellants. It is available as:
- 60 gram can (NDC 50222-302-60)
- 120 gram (2 cans of 60 gram) (NDC 50222-302-66)
16.2 Storage
- Store Enstilar Foam at 20°C - 25°C (68°F - 77°F); excursions permitted between 15°C - 30°C (59°F - 86°F). [See USP controlled room temperature].
- Contents under pressure. Do not puncture or incinerate. Do not expose to heat or store at temperatures above 120°F (49°C). Do not freeze.
- Unused product should be discarded six months after the can has been opened.
- Keep out of the reach of children.
17. Patient Counseling Information
See FDA-approved patient labeling (Patient Information and Instructions for Use).
| PATIENT INFORMATION ENSTILAR® [EN-still-ar] (calcipotriene and betamethasone dipropionate) Foam |
||
|---|---|---|
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Revised: 4/2022 | |
| Important: Enstilar Foam is for use on skin only (topical). Do not get Enstilar Foam near or in your mouth, eyes, or vagina. | ||
| There are other medicines that contain the same medicine that is in Enstilar Foam and are used to treat plaque psoriasis. Do not use other products containing calcipotriene or a corticosteroid medicine with Enstilar Foam without talking to your healthcare provider first. | ||
| What is Enstilar Foam?
Enstilar Foam is a prescription medicine used on the skin (topical) to treat plaque psoriasis in people 12 years of age and older. It is not known if Enstilar Foam is safe and effective in children under 12 years of age. |
||
Before you use Enstilar Foam, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. |
||
| How should I use Enstilar Foam? See the "Instructions for Use" for detailed information about the right way to use Enstilar Foam.
|
||
| What should I avoid while using Enstilar Foam? Enstilar Foam is flammable. Avoid fire, flame, and smoking when applying and right after you apply Enstilar Foam. |
||
| What are the possible side effects of Enstilar Foam? Enstilar Foam may cause serious side effects, including:
|
||
|
|
|
|
||
|
|
|
|
These are not all the possible side effects of Enstilar Foam. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
How should I store Enstilar Foam?
|
||
| General information about the safe and effective use of Enstilar Foam.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Enstilar Foam for a condition for which it was not prescribed. Do not give Enstilar Foam to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Enstilar Foam that is written for health professionals. |
||
| What are the ingredients in Enstilar Foam?
Active ingredients: calcipotriene and betamethasone dipropionate. Inactive ingredients: white petrolatum, polyoxypropylene stearyl ether, mineral oil, all-rac-alpha-tocopherol, and butylhydroxytoluene. Propellants: dimethyl ether and butane. Manufactured by: LEO Laboratories Ltd., 285 Cashel Road, Dublin 12, Ireland Distributed by: LEO Pharma Inc., Madison, NJ 07940, USA Enstilar® is a registered trademark of LEO Pharma A/S. © 2022, LEO Pharma Inc. All rights reserved. |
||
INSTRUCTIONS FOR USE ENSTILAR® [EN-still-ar] (calcipotriene and betamethasone dipropionate)Foam
This Instructions for Use contains information on how to apply Enstilar Foam.
Important Information You Need to Know Before Applying Enstilar Foam: Enstilar Foam is for use on skin only (topical). Do not get Enstilar Foam near or in your mouth, eyes or vagina. If you accidentally get Enstilar Foam on the face, in the mouth or in the eyes, wash the area with water right away. Do not swallow Enstilar Foam.
Applying Enstilar Foam:
Follow your healthcare provider's instructions on how much Enstilar Foam to use and where to use it.
|
| Wash your hands before applying Enstilar Foam. Step 1: Remove the cap from the can. Shake the can before use. |
|
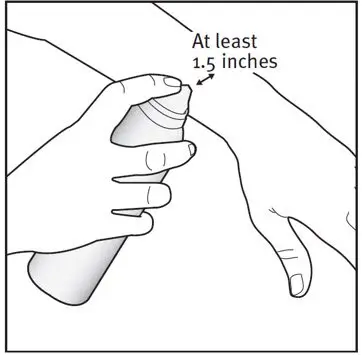
| Step 2: Hold the can at least 1.5 inches from the affected area. |
|
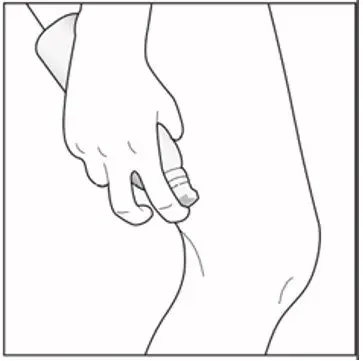
| Step 3: The foam can be sprayed holding the can in any position except sideways (horizontally). To spray, push down on the nozzle. Note: Enstilar Foam will slowly become smaller in size after spraying. |
|
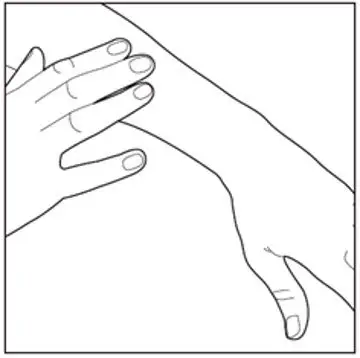
| Step 4: Gently rub in Enstilar Foam into your affected skin area. Repeat the steps above to apply Enstilar Foam to other affected areas as instructed by your healthcare provider. |
| Step 5: After applying Enstilar Foam, put the cap back on the can. | |
| Step 6: Wash your hands after using Enstilar Foam unless you are using the medicine to treat your hands. | |
Storing Enstilar Foam
- Store Enstilar Foam at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not expose Enstilar Foam to heat or store at temperatures above 120°F (49°C).
- Do not puncture or burn the Enstilar Foam can.
- Do not freeze Enstilar Foam.
Disposing of Enstilar Foam
- Enstilar Foam has an expiration date (exp.) marked on the can. Do not use after this date.
- Throw away (dispose of) unused Enstilar Foam 6 months after the can has been opened.
Keep Enstilar Foam and all medicines out of the reach of children.
Manufactured by:
LEO Laboratories Ltd.
285 Cashel Road
Dublin 12, Ireland
Distributed by:
LEO Pharma Inc.
Madison, NJ 07940, USA
Enstilar® is a registered trademark of LEO Pharma A/S.
© 2022, LEO Pharma Inc. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 4/2022
| ENSTILAR
calcipotriene and betamethasone dipropionate aerosol, foam |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - LEO Pharma Inc. (832692615) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LEO Laboratories Ltd. | 219532322 | ANALYSIS(50222-302) , MANUFACTURE(50222-302) , PACK(50222-302) , LABEL(50222-302) | |