Drug Class: Contraceptives Sex hormone combinations
Highlights of Prescribing Information
GENERESS Fe (norethindrone and ethinyl estradiol chewable tablets and ferrous fumarate chewable tablets)
Initial U.S. Approval: 1974
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
See full prescribing information for complete boxed warning.
• Women over 35 years old who smoke should not use GENERESS Fe. (4)
• Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use. (4)
Indications and Usage for Generess Fe
- GENERESS Fe is a combination of norethindrone, a progestin, and ethinyl estradiol, an estrogen, indicated for use by females of reproductive potential to prevent pregnancy. (1)
- The efficacy in females of reproductive potential with a body mass index (BMI) of > 35 kg/m2 has not been evaluated. (1, 8.8)
Generess Fe Dosage and Administration
- Chew one tablet without water at the same time every day. (2.1)
- Take tablets in the order directed on the blister pack. (2.1)
Dosage Forms and Strengths
GENERESS Fe consists of 28 tablets in the following order (3):
- 24 light green, round tablets (active) each containing 0.8 mg norethindrone and 0.025 mg ethinyl estradiol.
- 4 brown, round tablets (non-hormonal placebo) each containing 75 mg ferrous fumarate, which does not serve any therapeutic purpose.
Contraindications
- A high risk of arterial or venous thrombotic diseases. (4)
- Undiagnosed abnormal uterine bleeding. (4)
- Breast cancer (4)
- Liver tumors or liver disease (4)
- Co-administration with Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir (4)
Warnings and Precautions
- Vascular risks: Stop GENERESS Fe if a thrombotic event occurs. Stop at least 4 weeks before and through 2 weeks after major surgery. Start no earlier than 4 weeks after delivery in women who are not breastfeeding. (5.1)
- Liver disease: Discontinue if jaundice occurs. (5.3)
- High blood pressure: Do not prescribe for women with uncontrolled hypertension or hypertension with vascular disease. (5.5)
- Carbohydrate and lipid metabolic effects: Monitor prediabetic and diabetic women taking GENERESS Fe. Consider an alternate contraceptive method for women with uncontrolled dyslipidemia. (5.5)
- Headache: Evaluate significant change in headaches and discontinue if indicated. (5.8)
- Uterine bleeding: Evaluate irregular bleeding or amenorrhea. (5.9)
Adverse Reactions/Side Effects
The most common adverse reactions (≥ 2%) are nausea/vomiting (8.8%), headaches/migraine (7.5%), depression/mood complaints (4.1%), dysmenorrhea (3.9%), acne (3.2%), anxiety symptoms (2.4%), breast pain/tenderness (2.4%), and increased weight (2.3%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AbbVie at 1-800-678-1605 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Drugs or herbal products that induce certain enzymes, including CYP3A4, may decrease the effectiveness of COCs or increase breakthrough bleeding. Counsel patients to use a back-up method or alternative method of contraception when enzyme inducers are used with COCs. (7.1)
Use In Specific Populations
- Lactation: Not recommended, GENERESS Fe can decrease milk production. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2023
Full Prescribing Information
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs should not be used by women who are over 35 years of age and smoke [see Contraindications (4) and Warnings & Precautions (5.1)].
1. Indications and Usage for Generess Fe
GENERESS Fe is indicated for use by women to prevent pregnancy.
The efficacy of GENERESS Fe in women with a body mass index (BMI) of > 35 kg/m2 has not been evaluated.
2. Generess Fe Dosage and Administration
2.1 How to Take GENERESS Fe
To achieve maximum contraceptive effectiveness, GENERESS Fe must be taken exactly as directed. Chew and swallow one tablet without water at the same time every day. Tablets must be taken in the order directed on the blister pack. Tablets should not be skipped or taken at intervals exceeding 24 hours. GENERESS Fe may be administered without regard to meals [see Clinical Pharmacology (12.3)].
2.2 How to Start GENERESS Fe
Instruct the patient to begin taking GENERESS Fe on Day 1 of her menstrual cycle (that is, the first day of her menstrual bleeding). One light green tablet should be taken daily for 24 consecutive days followed by one brown tablet daily for 4 consecutive days. Instruct the patient to use a non-hormonal contraceptive as back-up during the first 7 days if she starts taking GENERESS Fe other than on the first day of her menstrual cycle.
For postpartum women who do not breastfeed or after a second trimester abortion, GENERESS Fe may be started no earlier than 4 weeks postpartum. Recommend use of a non-hormonal back-up method for the first 7 days. When combined oral contraceptives (COCs) are used during the postpartum period, the increased risk of thromboembolic disease associated with the postpartum period must be considered. The possibility of ovulation and conception before starting COCs should also be considered.
If the patient is switching from a combination hormonal method such as:
- Another pill
- Vaginal ring
- Patch
- Instruct her to take the first light green pill on the day she would have started a new cycle of her previous birth control pack (Day 1).
- If she previously used a vaginal ring or transdermal patch, she should start using GENERESS Fe on the day she would have restarted the ring or patch.
- Instruct the patient to use a non-hormonal back-up method such as a condom and spermicide for the first 7 days.
If the patient is switching from a progestin-only method such as:
- Progestin-only pill
- Implant
- Intrauterine system
- Injection
- Instruct her to take the first light green pill on the day she would have taken her next progestin-only pill or on the day of removal of her implant or intrauterine system or on the day when she would have had her next injection.
- Instruct the patient to use a non-hormonal back-up method such as a condom and spermicide for the first 7 days.
2.3 Missed Doses
| If one light green tablet is missed | Take the missed tablet as soon as possible. Take the next tablet at the regular time. Continue taking one tablet a day until the pack is finished. Additional nonhormonal contraception (such as condoms) is not needed. |
| If two light green tablets in a row are missed in Week 1 or Week 2 of the tablet pack | Take the two missed tablets as soon as possible, and the next two tablets the next day. Continue taking one tablet a day until the pack is finished. Use additional nonhormonal contraception (such as condoms) until hormonal tablets have been taken for 7 days after missing tablets. |
| If two light green tablets in a row are missed in Week 3 or Week 4 of the tablet pack | Throw away the remainder of the tablet pack. Start a new tablet pack the same day. Use additional nonhormonal contraception (such as condoms) until hormonal tablets have been taken for 7 days after missing tablets. |
| If three or more light green tablets in a row are missed | Throw away the missed tablets. Continue taking one tablet every day as indicated on the pack until the pack is finished. Bleeding may occur during the week following the missed tablets. Use additional nonhormonal contraception (such as condoms) until hormonal tablets have been taken for 7 days after missing tablets. |
| If any of the four brown tablets are missed | Throw away the missed tablets. Continue taking the remaining tablets until the pack is finished. Additional nonhormonal contraception (such as condoms) is not needed. |
3. Dosage Forms and Strengths
GENERESS Fe is available in blister packs.
Each blister pack (28 tablets) contains in the following order:
- 24 light green, round tablets (active) imprinted with “WC” on one side and “483” on the other and each containing 0.8 mg norethindrone and 0.025 mg ethinyl estradiol.
- 4 brown, round tablets (non-hormonal placebo) imprinted with “WC” on one side and “624” on the other and each containing 75 mg ferrous fumarate. The ferrous fumarate chewable tablets do not serve any therapeutic purpose.
4. Contraindications
GENERESS FE is contraindicated in females who are known to have or develop the following conditions:
- A high risk of arterial or venous thrombotic diseases. Examples include women who are known to:
- Smoke, if over age 35 [see Boxed Warning, and Warnings and Precautions (5.1)]
- Have deep vein thrombosis or pulmonary embolism, now or in the past [see Warnings and Precautions (5.1)]
- Have cerebrovascular disease [see Warnings and Precautions (5.1)]
- Have coronary artery disease [see Warnings and Precautions (5.1)]
- Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation) [see Warnings and Precautions (5.1)]
- Have inherited or acquired hypercoagulopathies [see Warnings and Precautions (5.1)]
- Have uncontrolled hypertension [see Warnings and Precautions (5.5)]
- Have diabetes with vascular disease [see Warnings and Precautions (5.7)]
- Have headaches with focal neurological symptoms or have migraine headaches with or without aura if over age 35 [see Warnings and Precautions (5.8)]
- Current diagnosis of, or history of, breast cancer, which may be hormone-sensitive [see Warnings and Precautions (5.2)]
- Liver tumors, benign or malignant, or liver disease [see Warnings and Precautions (5.3), Use in Specific Populations (8.7), and Clinical Pharmacology (12.3)]
- Undiagnosed abnormal uterine bleeding [see Warnings and Precautions (5.9)]
- Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations [see Warnings and Precautions (5.4)]
5. Warnings and Precautions
5.1 Thrombotic and Other Vascular Events
Stop GENERESS Fe if an arterial or deep venous thrombotic (VTE) event occurs. Although the use of COCs increases the risk of venous thromboembolism, pregnancy increases the risk of venous thromboembolism as much or more than the use of COCs. The risk of venous thromboembolism in women using COCs is 3 to 9 per 10,000 woman-years. The excess risk is highest during the first year of use of a COC. Use of COCs also increases the risk of arterial thromboses such as strokes and myocardial infarctions, especially in women with other risk factors for these events. The risk of thromboembolic disease due to oral contraceptives gradually disappears after COC use is discontinued.
If feasible, stop GENERESS Fe at least 4 weeks before and through 2 weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism.
Start GENERESS Fe no earlier than 4 weeks after delivery, in women who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the risk of ovulation increases after the third postpartum week.
COCs have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes), although, in general, the risk is greatest among older (> 35 years of age), hypertensive women who also smoke. COCs also increase the risk for stroke in women with other underlying risk factors.
Oral contraceptives must be used with caution in women with cardiovascular disease risk factors.
Stop GENERESS Fe if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
5.2 Malignant Neoplasms
Breast Cancer
GENERESS Fe is contraindicated in females who currently have or have had breast cancer because breast cancer may be hormonally sensitive [see Contraindications (4)].
Epidemiology studies have not found a consistent association between use of combined oral contraceptives (COCs) and breast cancer risk. Studies do not show an association between ever (current or past) use of COCs and risk of breast cancer. However, some studies report a small increase in the risk of breast cancer among current or recent users (<6 months since last use) and current users with longer duration of COC use [see Adverse Reactions (6.2)].
Cervical Cancer
Some studies suggest that COCs are associated with an increase in the risk of cervical cancer or intraepithelial neoplasia. However, there is controversy about the extent to which these findings may be due to differences in sexual behavior and other factors.
5.3 Liver Disease
Discontinue GENERESS Fe if jaundice develops. Steroid hormones may be poorly metabolized in patients with impaired liver function. Acute or chronic disturbances of liver function may necessitate the discontinuation of COC use until markers of liver function return to normal and COC causation has been excluded.
Hepatic adenomas are associated with COC use. An estimate of the attributable risk is 3.3 cases/100,000 COC users. Rupture of hepatic adenomas may cause death through intra-abdominal hemorrhage.
Studies have shown an increased risk of developing hepatocellular carcinoma in long-term (> 8 years) COC users. However, the attributable risk of liver cancers in COC users is less than one case per million users.
Oral contraceptive-related cholestasis may occur in women with a history of pregnancy-related cholestasis. Women with a history of COC-related cholestasis may have the condition recur with subsequent COC use.
5.4 Risk of Liver Enzyme Elevations with Concomitant Hepatitis C Treatment
During clinical trials with Hepatitis C combination drug regimen that contains ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, ALT elevations greater than 5 times the upper limit of normal (ULN), including some cases greater than 20 times the ULN, were significantly more frequent in women using ethinyl estradiol-containing medications, such as COCs. Discontinue GENERESS Fe prior to starting therapy with the combination drug regimen ombitasvir/paritaprevir/ritonavir, with or without dasabuvir [see Contraindications (4)]. GENERESS Fe can be restarted approximately 2 weeks following completion of treatment with the Hepatitis C combination drug regimen.
5.5 High Blood Pressure
For women with well-controlled hypertension, monitor blood pressure and stop GENERESS Fe if blood pressure rises significantly. Women with uncontrolled hypertension or hypertension with vascular disease should not use COCs.
An increase in blood pressure has been reported in women taking COCs, and this increase is more likely in older women and with extended duration of use. The incidence of hypertension increases with increasing concentration of progestin.
5.7 Carbohydrate and Lipid Metabolic Effects
Carefully monitor prediabetic and diabetic women who are taking GENERESS Fe. COCs may decrease glucose tolerance in a dose-related fashion.
Consider alternative contraception for women with uncontrolled dyslipidemia. A small proportion of women will have adverse lipid changes while on COCs.
Women with hypertriglyceridemia, or a family history thereof, may be at an increased risk of pancreatitis when using COCs.
5.8 Headache
If a woman taking GENERESS Fe develops new headaches that are recurrent, persistent, or severe, evaluate the cause and discontinue GENERESS Fe if indicated.
An increase in frequency or severity of migraine during COC use (which may be prodromal of a cerebrovascular event) may be a reason for immediate discontinuation of the COC.
5.9 Bleeding Irregularities
Unscheduled (breakthrough or intracyclic) bleeding and spotting sometimes occur in patients on COCs, especially during the first three months of use. If bleeding persists or occurs after previously regular cycles, check for causes such as pregnancy or malignancy. If pathology and pregnancy are excluded, bleeding irregularities may resolve over time or with a change to a different COC.
Patient diaries from the clinical trial of GENERESS Fe showed that on the first cycle of use, 37% of subjects taking GENERESS Fe had unscheduled bleeding and/or spotting. From Cycle 2-13, the percent of women with unscheduled bleeding/spotting ranged from 21-31% per cycle. For those women with unscheduled bleeding/spotting, the mean number of days of unscheduled bleeding/spotting was 5.2 in the first cycle of use and ranged from 3.6 – 4.2 in cycles 2-13. A total of 15 subjects out of 1,677 (0.9%) discontinued the study prematurely due to metrorrhagia or irregular menstruation.
Women who are not pregnant and use GENERESS Fe may not have scheduled (withdrawal) bleeding every cycle or may experience amenorrhea (absence of any bleeding and spotting). The incidence of amenorrhea in the clinical trial increased from 8.1% of the subjects in Cycle 2 to 18.4% by Cycle 13. For those women who had scheduled (withdrawal) bleeding, the average duration of bleeding per cycle in Cycles 2-13 was 3.7 days.
If the patient has not adhered to the prescribed dosing schedule (missed one or more active tablets or started taking them on a day later than she should have), consider the possibility of pregnancy at the time of the first missed period and take appropriate diagnostic measures. If the patient has adhered to the prescribed regimen and misses two consecutive periods, rule out pregnancy.
Some women may encounter amenorrhea or oligomenorrhea after stopping COCs, especially when such a condition was pre-existent.
6. Adverse Reactions/Side Effects
The following serious adverse reactions with the use of COCs are discussed elsewhere in the labeling:
- Serious cardiovascular events and smoking [see Boxed Warning, and Warnings and Precautions (5.1)]
- Vascular events [see Warnings and Precautions (5.1)]
- Liver disease [see Warnings and Precautions (5.3)]
Adverse reactions commonly reported by COC users are:
- Irregular uterine bleeding
- Nausea
- Breast tenderness
- Headache
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
A phase 3 clinical trial evaluated the safety and efficacy of GENERESS Fe for pregnancy prevention. The study was a multicenter, non-comparative, open-label study with a treatment duration of 12 months (thirteen 28-day cycles). A total of 1,677 women aged 18-46 were enrolled and took at least one dose of GENERESS Fe.
Adverse Reactions Leading to Study Discontinuation: 8.5% of the women discontinued from the clinical trial due to an adverse reaction. The most common adverse reactions leading to discontinuation were nausea (1.0%), weight increase (0.8%), acne (0.8%), metrorrhagia (0.7%), altered mood (0.4%), hypertension (0.4%), irritability (0.3%), migraine (0.3%), decreased libido (0.3%) and mood swings (0.3%).
Common Adverse Reactions (≥ 2% of all treated subjects): nausea/vomiting (8.8%), headaches/migraine (7.5%), depression/mood complaints (4.1%), dysmenorrhea (3.9%), acne (3.2%), anxiety symptoms (2.4%), breast pain/tenderness (2.4%), and increased weight (2.3%).
Serious Adverse Reactions: Hypertension, depression, cholecystitis, and deep vein thrombosis.
6.2 Postmarketing Experience
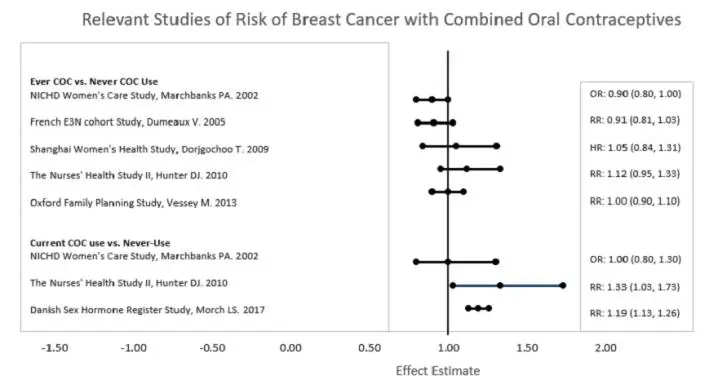
Five studies that compared breast cancer risk between ever-users (current or past use) of COCs and never-users of COCs reported no association between ever use of COCs and breast cancer risk, with effect estimates ranging from 0.90 – 1.12 (Figure 1).
Three studies compared breast cancer risk between current or recent COC users (<6 months since last use) and never users of COCs (Figure 1). One of these studies reported no association between breast cancer risk and COC use. The other two studies found an increased relative risk of 1.19 – 1.33 with current or recent use. Both of these studies found an increased risk of breast cancer with current use of longer duration, with relative risks ranging from 1.03 with less than one year of COC use to approximately 1.4 with more than 8-10 years of COC use.
Figure 1.
RR = relative risk; OR = odds ratio; HR = hazard ratio. “ever COC” are females with current or past COC use; “never COC use” are females that never used COCs.
7. Drug Interactions
No drug-drug interaction studies were conducted with GENERESS Fe.
7.1 Changes in Contraceptive Effectiveness Associated with Co-Administration of Other Products
If a woman on hormonal contraceptives takes a drug or herbal product that induces enzymes, including CYP3A4, that metabolize contraceptive hormones, counsel her to use additional contraception or a different method of contraception. Drugs or herbal products that induce such enzymes may decrease the plasma concentrations of contraceptive hormones, and may decrease the effectiveness of hormonal contraceptives or increase breakthrough bleeding. Some drugs or herbal products that may decrease the effectiveness of hormonal contraceptives include:
- barbiturates
- bosentan
- carbamazepine
- felbamate
- griseofulvin
- oxcarbazepine
- phenytoin
- rifampin
- St. John’s wort
- topiramate
HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors: Significant changes (increase or decrease) in the plasma levels of the estrogen and progestin have been noted in some cases of co-administration of HIV protease inhibitors or with non-nucleoside reverse transcriptase inhibitors.
Antibiotics: There have been reports of pregnancy while taking hormonal contraceptives and antibiotics, but clinical pharmacokinetic studies have not shown consistent effects of antibiotics on plasma concentrations of synthetic steroids.
Consult the labeling of all concurrently-used drugs to obtain further information about interactions with hormonal contraceptives or the potential for enzyme alterations.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There is no use for contraception in pregnancy; therefore, GENERESS Fe should be discontinued during pregnancy. Epidemiologic studies and meta-analyses have not found an increased risk of genital or nongenital birth defects (including cardiac anomalies and limb-reduction defects) following exposure to COCs before conception or during early pregnancy.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4 percent and 15 to 20 percent, respectively.
8.2 Lactation
Risk Summary
Contraceptive hormones and/or metabolites are present in human milk. COCs can reduce milk production in breast-feeding females. This reduction can occur at any time but is less likely to occur once breast-feeding is well-established. When possible, advise the nursing female to use other methods of contraception until she discontinues breast-feeding [see Dosage and Administration (2.2)]. The developmental and health benefits of breast-feeding should be considered along with the mother’s clinical need for GENERESS Fe and any potential adverse effects on the breast-fed child from GENERESS Fe or from the underlying maternal condition.
8.7 Hepatic Impairment
No studies have been conducted to evaluate the effect of hepatic disease on the disposition of GENERESS Fe. However, steroid hormones may be poorly metabolized in patients with impaired liver function. Acute or chronic disturbances of liver function may necessitate the discontinuation of COC use until markers of liver function return to normal [see Contraindications (4), and Warnings and Precautions (5.3)].
12. Generess Fe - Clinical Pharmacology
12.3 Pharmacokinetics
Absorption
Norethindrone and ethinyl estradiol are absorbed with maximum plasma concentrations occurring within 2 hours after GENERESS Fe administration (see Table 1). Both are subject to first-pass metabolism after oral dosing, resulting in an absolute bioavailability of approximately 64% for norethindrone and 43% for ethinyl estradiol.
The plasma norethindrone and ethinyl estradiol pharmacokinetics following single- and multiple-dose administrations of GENERESS Fe in 17 healthy female volunteers are provided in Table 1.
Following multiple-dose administration of GENERESS Fe, mean maximum concentrations of norethindrone and ethinyl estradiol were increased by 126% and 14%, respectively, as compared to single-dose administration. Mean norethindrone and ethinyl estradiol exposures (AUC values) were increased by 239% and 55% respectively, as compared to single-dose administration of GENERESS Fe.
Mean sex hormone binding globulin (SHBG) concentrations were increased by 170% from baseline (40.0 pg/mL; CV = 65%) to 108 pg/mL (CV = 45%) at steady-state.
| Arithmetic mean parameters (%CV) | |||||
| Regimen | Analyte | Cmax | tmax | AUC0 - 24h | t1/2 * |
| Day 1 (Single Dose) N = 17 | NE | 9,840 (36) | 1.4 (49) | 41,680 (47) | |
| EE | 147 (25) | 1.2 (27) | 903 (18) | ||
| Day 24 (Multiple Dose) N = 17 | NE | 22,200 (30) | 1.6 (76) | 141,200 (32) | 10.8 |
| EE | 168 (25) | 1.2 (35) | 1,400 (32) | 17.1 | |
* The harmonic mean for t1/2 is presented
EE = ethinyl estradiol; NE = norethindrone
%CV = coefficient of variation; Cmax = maximum plasma concentration (pg/mL); tmax = time of the maximum measured plasma concentration (h); AUC0-24h = area under the plasma concentration versus time curve from time 0 to 24h (pg•h/mL); t1/2 = apparent elimination half life (h)
Food Effect
GENERESS Fe may be administered with or without food. A single-dose administration of GENERESS Fe with food decreased the maximum concentration of norethindrone by 47% and increased the extent of absorption by 10-14% and decreased the maximum concentration of ethinyl estradiol by 39% but not the extent of absorption.
Distribution
Volume of distribution of norethindrone and ethinyl estradiol ranges from 2 to 4 L/kg. Plasma protein binding of both steroids is extensive (> 95%); norethindrone binds to both albumin and SHBG, whereas ethinyl estradiol binds only to albumin. Although ethinyl estradiol does not bind to SHBG, it induces SHBG synthesis.
Metabolism
Norethindrone undergoes extensive biotransformation, primarily via reduction, followed by sulfate and glucuronide conjugation. The majority of metabolites in the circulation are sulfates, with glucuronides accounting for most of the urinary metabolites. A small amount of norethindrone is metabolically converted to ethinyl estradiol, such that exposure to ethinyl estradiol following administration of 1 mg of norethindrone acetate is equivalent to oral administration of 2.8 mcg ethinyl estradiol; therefore 0.8 mg norethindrone would be equivalent to the oral administration of 2.6 mcg ethinyl estradiol.
Ethinyl estradiol is also extensively metabolized, both by oxidation and by conjugation with sulfate and glucuronide. Sulfates are the major circulating conjugates of ethinyl estradiol and glucuronides predominate in urine. The primary oxidative metabolite is 2-hydroxy ethinyl estradiol, formed by the CYP3A4 isoform of cytochrome P450. Part of the first-pass metabolism of ethinyl estradiol is believed to occur in gastrointestinal mucosa. Ethinyl estradiol may undergo enterohepatic circulation.
Excretion
Norethindrone and ethinyl estradiol are excreted in both urine and feces, primarily as metabolites. Plasma clearance values for norethindrone and ethinyl estradiol are similar (approximately 0.4 L/hr/kg). Elimination half-lives of norethindrone and ethinyl estradiol following administration of 0.8 mg norethindrone / 0.025 mcg ethinyl estradiol tablets are approximately 11 hours and 17 hours, respectively.
Specific Populations
Pediatric Use: Safety and efficacy of GENERESS Fe have been established in women of reproductive age. Efficacy is expected to be the same for postpubertal adolescents under the age of 18 and for users 18 years and older. Use of this product before menarche is not indicated.
Geriatric Use: GENERESS Fe has not been studied in postmenopausal women and is not indicated in this population.
Renal Impairment: The pharmacokinetics of GENERESS Fe have not been studied in subjects with renal impairment.
Hepatic Impairment: The pharmacokinetics of GENERESS Fe have not been studied in subjects with hepatic impairment. Steroid hormones may be poorly metabolized in patients with impaired liver function. Acute or chronic disturbances of liver function may necessitate the discontinuation of COC use until markers of liver function return to normal [see Contraindications (4), and Warnings and Precautions (5.3)].
Body Mass Index: The efficacy of GENERESS Fe in women with a BMI of > 35 kg/m2 has not been evaluated.
FDA-Approved Patient Labeling
Guide for Using GENERESS Fe (norethindrone and ethinyl estradiol chewable tablets and ferrous fumarate chewable tablets)
| WARNING TO WOMEN WHO SMOKE |
| Do not use GENERESS Fe if you smoke cigarettes and are over 35 years old. Smoking increases your risk of serious cardiovascular side effects (heart and blood vessel problems) from birth control pills, including death from heart attack, blood clots or stroke. This risk increases with age and the number of cigarettes you smoke. |
Birth control pills help to lower the chances of becoming pregnant when taken as directed. They do not protect against HIV infection (AIDS) and other sexually transmitted diseases.
What is GENERESS Fe?
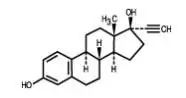
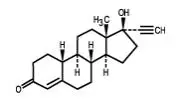
GENERESS Fe is a birth control pill. It contains two female hormones, an estrogen called ethinyl estradiol and a progestin called norethindrone.
How Do I Take GENERESS Fe?
- Chew and swallow one pill every day without water at the same time. Take the pills in the order directed on the blister pack.
- Do not skip pills or take at intervals exceeding 24 hours. If you miss pills (including starting the pack late), you could get pregnant. The more pills you miss, the more likely you are to get pregnant.
- If you have trouble remembering to take GENERESS Fe, talk to your healthcare provider about how to make pill‑taking easier or about using another method of birth control.
- You may have spotting or light bleeding when you first take GENERESS Fe. Spotting or light bleeding is normal at first.
- You may feel sick to your stomach (nauseated), especially during the first few months that you take GENERESS Fe. If you feel sick to your stomach, do not stop taking the pill. The problem will usually go away. If your nausea doesn't go away, call your healthcare provider.
- If you vomit or have diarrhea within 3-4 hours of taking your pill, follow the instructions for “What Should I Do if I Miss any Pills.”
- Missing pills can also cause spotting or light bleeding, even when you take the missed pills late. On the days you take 2 pills to make up for missed pills, you could also feel a little sick to your stomach.
How Well Does GENERESS Fe Work?
Your chance of getting pregnant depends on how well you follow the directions for taking your birth control pills. The more carefully you follow the directions, the less chance you have of getting pregnant.
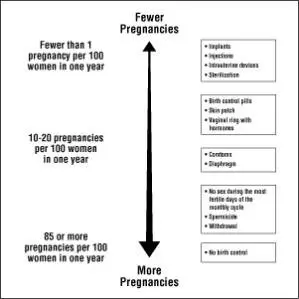
Based on the results of one clinical study, 1 to 3 women out of 100 women may get pregnant during the first year they use GENERESS Fe.
The following chart shows the chance of getting pregnant for women who use different methods of birth control. Each box on the chart contains a list of birth control methods that are similar in effectiveness. The most effective methods are at the top of the chart. The box on the bottom of the chart shows the chance of getting pregnant for women who do not use birth control and are trying to get pregnant.
Before you start taking GENERESS Fe
- Decide what time of day to take your pill. It is important to take it at the same time every day and in the order as directed on the blister pack.
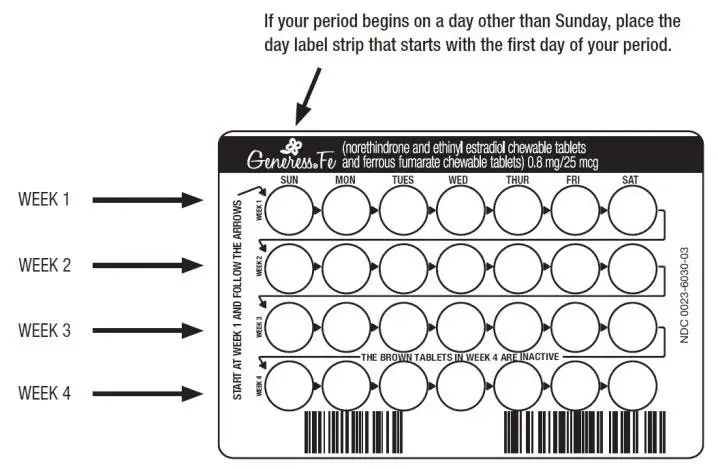
- Look at your GENERESS Fe blister pack. The blister pack has four rows of 7 pills each, for a total of 28 pills. Find:
- where on the pack to start taking your pills
- in what order to take the pills
Each GENERESS Fe blister pack has 28 pills
- 24 light green pills with hormones for Weeks 1, 2 and 3 and the first part of Week 4
- 4 brown pills without hormones for the remainder of Week 4
- Be sure to have ready at all times another kind of birth control (such as a condom and spermicide) to use as a back-up in case you miss pills.
When to Start GENERESS Fe
If you start taking GENERESS Fe and you did not use a hormonal birth control method before:
DAY-1 START:
- Pick the day label strip that starts with the first day of your period (this is the day you start bleeding or spotting, even if it is almost midnight when the bleeding begins). Pick a time of day that will be easy to remember.
- Place this day label strip on the tablet dispenser over the area that has the days of the week (starting with Sunday) printed on the plastic.
- Take the first light green pill of the first pack during the first 24 hours of your period.
- You will not need to use a back-up method of birth control because you are starting the pill at the beginning of your period. However, if you start on a day other than the first day of your period or if you are starting after having been pregnant and have not yet had a period, use a back-up method of birth control such as a condom and spermicide until you have taken a light green pill for 7 days in a row.
- After taking the last brown pill (day 28) of the blister pack, start taking the first light green pill from a new blister pack the very next day whether or not you are having your period.
If you start taking GENERESS Fe and you are switching from a combination hormonal method such as:
- another pill
- vaginal ring
- patch
- Take the first light green pill on the first day you would have started your previous birth control pack.
- If you previously used a vaginal ring or transdermal patch, finish the 21 days of use and wait 7 days after removal of the ring or transdermal patch before starting GENERESS Fe.
- Use a non-hormonal back-up method such as a condom and spermicide for the first 7 days you take GENERESS Fe.
If you start taking GENERESS Fe and you are switching from a progestin-only method such as a:
- progestin-only pill
- implant
- intrauterine system
- injection
- Take the first light green pill on the day you would have taken your next progestin-only pill or on the day of removal of your implant or intrauterine system or on the day when you would have had your next injection.
- Use a non-hormonal back-up method such as a condom and spermicide for the first 7 days you take GENERESS Fe.
What Should I do if I Miss any Pills
If you forgot to start a new blister pack, you may already be pregnant. Use back-up contraception (such as a condom and spermicide) anytime you have sex. Call your healthcare provider if you are unsure whether you are pregnant.
Your birth control pills may not be as effective if you miss any light green pills, and particularly if you miss the first few or the last few light green pills in a pack.
If you MISS ONE light green pill
- Take it as soon as you remember. Take the next pill at your regular time. This means you may take two pills in 1 day.
- You do not need to use a back‑up birth control method if you have sex.
If you MISS TWO light green pills in a row in WEEK 1 or WEEK 2 of your pack
- Take two pills on the day you remember and two pills the next day.
- Then take one pill a day until you finish the pack.
- You could become pregnant if you have sex during the 7 days after you restart your pills. You MUST use a non-hormonal birth control method (such as a condom and spermicide) as a back‑up for those 7 days.
If you MISS TWO light green pills in a row in WEEK 3 or WEEK 4 of your pack
- THROW OUT the rest of the pill pack and start a new pack that same day.
- You could become pregnant if you have sex during the 7 days after you restart your pills. You MUST use a non-hormonal birth control method (such as a condom and spermicide) as a back‑up for those 7 days after you restart your pills.
If you MISS THREE OR MORE light green pills in a row at any time
- THROW OUT the rest of the pill pack and start a new pack that same day.
- You could become pregnant if you have sex on the days when you missed pills or during the first 7 days after restarting your pills. You MUST use a non-hormonal birth control method (such as a condom and spermicide) as a back‑up the next time you have sex and for the first 7 days after you restart your pills.
If you forget any of the four brown "reminder" pills in WEEK 4
- THROW AWAY the pills you missed.
- Keep taking one pill each day until the pack is finished.
- You do not need to use a back‑up method of birth control.
| You may already be pregnant or COULD BECOME PREGNANT if you had sex on the days after the pills were missed. The more pills missed and the closer they are to the end of the cycle, the higher the risk of a pregnancy. You should call your doctor or healthcare provider if you are unsure whether you are already pregnant. |
If you are still not sure of what to do about the pills you have missed:
- Call your healthcare provider.
- Use a back-up contraception (such as a condom and spermicide) anytime you have sex and keep taking 1 pill each day.
Who Should not Take GENERESS Fe?
Your healthcare provider will not give you GENERESS Fe if you have:
- Ever had breast cancer, which may be sensitive to female hormones
- Liver disease, including liver tumors
- Take any Hepatitis C drug combination containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir. This may increase levels of the liver enzyme “alanine aminotransferase” (ALT) in the blood.
- Ever had blood clots in your arms, legs, or lungs
- Ever had a stroke
- Ever had a heart attack
- Certain heart valve problems or heart rhythm abnormalities that can cause blood clots to form in the heart
- An inherited problem with your blood that makes it clot more than normal
- High blood pressure that medicine can't control
- Diabetes with kidney, eye, or blood vessel damage
- Certain kinds of severe migraine headaches with aura, numbness, weakness or changes in vision
Also, do not take birth control pills if you:
- Smoke and are over 35 years old
- Are pregnant
Birth control pills may not be a good choice for you if you have ever had jaundice (yellowing of the skin or eyes) caused by pregnancy (also called cholestasis of pregnancy).
What Else Should I Know about Taking GENERESS Fe?
Birth control pills do not protect you against any sexually transmitted disease, including HIV, the virus that causes AIDS.
Do not skip any pills, even if you do not have sex often.
If you miss a period, you could be pregnant. However, some women miss periods or have light periods on birth control pills, even when they are not pregnant. Contact your healthcare provider for advice if you:
- Think you are pregnant
- Miss one period and have not taken your birth control pills according to directions
- Miss two periods in a row
Birth control pills should not be taken during pregnancy. However, birth control pills taken by accident during pregnancy are not known to cause birth defects.
You should stop GENERESS Fe at least four weeks before you have major surgery and not restart it until at least two weeks after the surgery due to an increased risk of blood clots.
If you are breastfeeding, consider another birth control method until you are ready to stop breastfeeding. Birth control pills that contain estrogen, like GENERESS Fe, may decrease the amount of milk you make. A small amount of the pill's hormones pass into breast milk.
Tell your healthcare provider about all medicines and herbal products that you take. Some medicines and herbal products may make birth control pills less effective, including:
- barbiturates
- bosentan
- carbamazepine
- felbamate
- griseofulvin
- oxcarbazepine
- phenytoin
- rifampin
- St. John’s wort
- topiramate
Consider using another birth control method when you take medicines that may make birth control pills less effective.
Birth control pills may interact with lamotrigine, an anticonvulsant used for epilepsy. This may increase the risk of seizures, so your healthcare provider may need to adjust the dose of lamotrigine.
If you have vomiting or diarrhea, your birth control pills may not work as well. Use another birth control method, like a condom and spermicide, until you check with your healthcare provider.
What are the Most Serious Risks of Taking Birth Control Pills?
Like pregnancy, birth control pills increase the risk of serious blood clots, especially in women who have other risk factors, such as smoking, obesity, or age greater than 35. It is possible to die from a problem caused by a blood clot, such as a heart attack or a stroke. Some examples of serious blood clots are blood clots in the:
- Legs (thrombophlebitis)
- Lungs (pulmonary embolus)
- Eyes (loss of eyesight)
- Heart (heart attack)
- Brain (stroke)
A few women who take birth control pills may get:
- High blood pressure
- Gallbladder problems
- Rare cancerous or noncancerous liver tumors
All of these events are uncommon in healthy women.
Call your healthcare provider right away if you have:
- Persistent leg pain
- Sudden shortness of breath
- Sudden blindness, partial or complete
- Severe pain in your chest
- Sudden, severe headache unlike your usual headaches
- Weakness or numbness in an arm or leg, or trouble speaking
- Yellowing of the skin or eyeballs
What are the Common Side Effects of Birth Control Pills?
The most common side effects of birth control pills are:
- Spotting or bleeding between menstrual periods
- Nausea
- Breast tenderness
- Headache
These side effects are usually mild and usually disappear with time.
Less common side effects are:
- Acne
- Less sexual desire
- Bloating or fluid retention
- Blotchy darkening of the skin, especially on the face
- High blood sugar, especially in women who already have diabetes
- High fat levels in the blood
- Depression, especially if you have had depression in the past. Call your healthcare provider immediately if you have any thoughts of harming yourself.
- Problems tolerating contact lenses
- Weight changes
This is not a complete list of possible side effects. Talk to your healthcare provider if you develop any side effects that concern you. You may report side effects to the FDA at 1-800-FDA-1088.
No serious problems have been reported from a birth control pill overdose, even when accidentally taken by children.
Do Birth Control Pills Cause Cancer?
It is not known if hormonal birth control pills cause breast cancer. Some studies, but not all, suggest that there could be a slight increase in the risk of breast cancer among current users with longer duration of use.
If you have breast cancer now, or have had it in the past, do not use hormonal birth control because some breast cancers are sensitive to hormones.
Women who use birth control pills may have a slightly higher chance of getting cervical cancer. However, this may be due to other reasons such as having more sexual partners.
What Should I Know about My Period when Taking GENERESS Fe?
Unscheduled (irregular) vaginal bleeding or spotting may occur while you are taking GENERESS Fe. Unscheduled bleeding may vary from slight staining to breakthrough bleeding, which is a flow much like a regular period, but which occurs between menstrual periods. Unscheduled bleeding occurs most often during the first few months of oral contraceptive use, but may also occur after you have been taking the pill for some time. Such bleeding may be temporary and usually does not indicate any serious problems.
Approximately one-third of the women who use GENERESS Fe have unscheduled bleeding or spotting in the first months of use. About one-quarter of users continue to have unscheduled bleeding or spotting after one year of use.
It is important to continue taking your pills on schedule. If the bleeding occurs in more than one cycle, is unusually heavy, or lasts for more than a few days, call your healthcare provider.
What if I Miss My Scheduled Period when Taking GENERESS Fe?
Women who use GENERESS Fe may not have a period at the end of every 28-day pack of pills.
If you miss more than two periods in a row or miss one period when you have not taken your birth control pills according to directions, call your healthcare provider. Also notify your healthcare provider if you have symptoms of pregnancy such as morning sickness or unusual breast tenderness. It is important that your healthcare provider checks you to find out if you are pregnant. Stop taking GENERESS Fe if you are pregnant.
What If I Want to Become Pregnant?
You may stop taking the pill whenever you wish. Consider a visit with your healthcare provider for a pre-pregnancy checkup before you stop taking the pill.
General Advice about GENERESS Fe
Your healthcare provider prescribed GENERESS Fe for you. Please do not share GENERESS Fe with anyone else. Keep GENERESS Fe out of the reach of children.
If you have concerns or questions, ask your healthcare provider. You may also ask your healthcare provider for a more detailed label written for medical professionals.
For more information, go to www.generess.com or you can contact AbbVie at 1-800-678-1605.
For all medical inquiries contact:
AbbVie Inc.
1 N Waukegan Road
North Chicago, IL 60064
1-800-678-1605
Distributed By:
AbbVie Inc.
1 N Waukegan Road
North Chicago, IL 60064
© 2023 AbbVie. All rights reserved.
Generess and its design are trademarks of Allergan Pharmaceuticals International Limited, an AbbVie company.
Revised: 06/2023
V3.0PPI6030
| GENERESS FE
norethindrone and ethinyl estradiol and ferrous fumarate kit |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Allergan, Inc. (144796497) |