Drug Detail:Gimoti (Metoclopramide (nasal spray))
Drug Class: GI stimulants
Highlights of Prescribing Information
GIMOTI™ (metoclopramide) nasal spray
Initial U.S. Approval: 1979
WARNING: TARDIVE DYSKINESIA
See full prescribing information for complete boxed warning.
- Metoclopramide can cause tardive dyskinesia (TD), a serious movement disorder that is often irreversible. The risk of developing TD increases with duration of treatment and total cumulative dosage. (5.1)
- Discontinue GIMOTI in patients who develop signs or symptoms of TD. (5.1)
- Avoid treatment with metoclopramide (all dosage forms and routes of administration) for longer than 12 weeks because of the risk of developing TD with longer-term use. (5.1, 2.1)
Indications and Usage for Gimoti
GIMOTI is a dopamine-2 (D2) antagonist indicated for the relief of symptoms in adults with acute and recurrent diabetic gastroparesis. (1)
Limitations of Use:
GIMOTI is not recommended for use in:
- pediatric patients due to the risk of tardive dyskinesia (TD) and other extrapyramidal symptoms as well as the risk of methemoglobinemia in neonates. (1, 8.4)
- moderate or severe hepatic impairment (Child-Pugh B or C), moderate or severe renal impairment (creatinine clearance less than 60 mL/minute), and patients concurrently using strong CYP2D6 inhibitors due to the risk of increased drug exposure and adverse reactions. (1, 5.9, 7.1)
Gimoti Dosage and Administration
Administration
- See the full prescribing information for complete information on administration. (2.1)
Recommended Dosage
- Adults less than 65 years of age: The recommended dosage is 1 spray (15 mg) in one nostril, 30 minutes before each meal and at bedtime (maximum of 4 sprays daily) for 2 to 8 weeks, depending on symptomatic response. (2.2)
- Adults 65 years of age and older: GIMOTI is not recommended in geriatric patients as initial therapy. Geriatric patients receiving an alternative metoclopramide product at a stable dosage of 10 mg four times daily can be switched to GIMOTI 1 spray (15 mg) in one nostril, 30 minutes before each meal and at bedtime (maximum four times daily) for 2 to 8 weeks, depending on symptomatic response. (2.2, 8.5)
Dosage Forms and Strengths
Nasal Spray: 15 mg metoclopramide in each 70 microliter spray. (3)
Contraindications
- History of TD or dystonic reaction to metoclopramide (4)
- When stimulation of gastrointestinal motility might be dangerous (4)
- Pheochromocytoma, catecholamine-releasing paragangliomas (4)
- Epilepsy (4)
- Hypersensitivity to metoclopramide (4)
Warnings and Precautions
- Tardive dyskinesia (TD), other extrapyramidal symptoms (EPS), and neuroleptic malignant syndrome (NMS): Avoid concomitant use of other drugs known to cause TD/EPS/NMS and avoid use in patients with Parkinson's disease. If symptoms occur, discontinue GIMOTI and seek immediate medical attention. (5.1, 5.2, 5.3, 7.1, 7.2)
- Depression and suicidal ideation/suicide: Avoid use. (5.4)
Adverse Reactions/Side Effects
Most common adverse reactions (≥5%) are: dysgeusia, headache, and fatigue. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Evoke Pharma, Inc. at 1-833-4-GIMOTI (1-833-444-6684), or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Antipsychotics: Potential for additive effects, including TD, EPS, and NMS; avoid concomitant use. (7.1)
- Central nervous system (CNS) depressants: Increased risk of CNS depression. Avoid concomitant use and monitor for adverse reactions. (7.1)
- Monoamine oxidase (MAO) inhibitors: Increased risk of hypertension; avoid concomitant use. (5.5, 7.1)
- Additional drug interactions: See Full Prescribing Information. (7.1, 7.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2021
Related/similar drugs
metoclopramide, Reglan, cisapride, Metozolv ODT, PropulsidFull Prescribing Information
WARNING: TARDIVE DYSKINESIA
- Metoclopramide can cause tardive dyskinesia (TD), a serious movement disorder that is often irreversible. The risk of developing TD increases with duration of treatment and total cumulative dosage [see Warnings and Precautions (5.1)].
- Discontinue GIMOTI in patients who develop signs or symptoms of TD. In some patients, symptoms may lessen or resolve after metoclopramide is stopped [see Warnings and Precautions (5.1)].
- Avoid treatment with metoclopramide (all dosage forms and routes of administration) for longer than 12 weeks because of the increased risk of developing TD with longer-term use [see Warnings and Precautions (5.1) and Dosage and Administration (2.1)].
1. Indications and Usage for Gimoti
GIMOTI is indicated for the relief of symptoms in adults with acute and recurrent diabetic gastroparesis.
2. Gimoti Dosage and Administration
2.1 Important Administration and Storage Instructions
- Avoid treatment with metoclopramide (all dosage forms and routes of administration) for longer than 12 weeks because of the increased risk of developing TD with longer-term use [see Warnings and Precautions (5.1)].
- One spray in one nostril administers the appropriate dose.
- Before administering the first dose from a bottle, prime the pump by pressing down on the finger flange and releasing 10 sprays in the air.
- Place the spray nozzle tip under one nostril and lean the head slightly forward so the tip of spray nozzle is aimed away from the septum and toward the back of the nose.
- Close the other nostril with the other index finger. Move spray pump upwards so the tip of the nozzle is in the nostril.
- To ensure a full dose, hold the bottle upright while pressing down firmly and completely on finger flange and release while inhaling slowly through the open nostril.
- Remove spray pump nozzle tip from nostril and exhale slowly through the mouth.
- Wipe the spray nozzle with a clean tissue.
3. Dosage Forms and Strengths
Nasal Spray: 15 mg of metoclopramide in each 70 microliter spray. GIMOTI is an aqueous solution supplied in an amber glass bottle fitted with a metered spray pump attachment.
4. Contraindications
GIMOTI is contraindicated:
- In patients with a history of tardive dyskinesia (TD) or a dystonic reaction to metoclopramide [see Warnings and Precautions (5.1, 5.2)].
- When stimulation of gastrointestinal motility might be dangerous (e.g., in the presence of gastrointestinal hemorrhage, mechanical obstruction, or perforation).
- In patients with pheochromocytoma or other catecholamine-releasing paragangliomas. Metoclopramide may cause a hypertensive/pheochromocytoma crisis, probably due to release of catecholamines from the tumor [see Warnings and Precautions (5.5)].
- In patients with epilepsy. Metoclopramide may increase the frequency and severity of seizures [see Adverse Reactions (6)].
- In patients with hypersensitivity to metoclopramide. Reactions have included laryngeal and glossal angioedema and bronchospasm [see Adverse Reactions (6)].
5. Warnings and Precautions
5.1 Tardive Dyskinesia
Metoclopramide can cause tardive dyskinesia (TD), a syndrome of potentially irreversible and disfiguring involuntary movements of the face or tongue, and sometimes of the trunk and/or extremities. Movements may be choreoathetotic in appearance. The risk of developing TD and the likelihood that TD will become irreversible increases with duration of treatment and total cumulative dosage. Additionally, the risk of developing TD is increased among the elderly, especially elderly women [see Use in Specific Populations (8.5)], and in patients with diabetes mellitus. Due to the risk of developing TD, avoid treatment with metoclopramide for longer than 12 weeks. GIMOTI is not recommended in geriatric patients as initial therapy [see Dosage and Administration (2.2)].
Discontinue GIMOTI immediately in patients who develop signs and symptoms of TD. Consider treatment for established cases of TD, although in some patients TD may remit, partially or completely, within several weeks to months after GIMOTI is withdrawn.
Metoclopramide itself may suppress, or partially suppress, the signs of TD, thereby masking the underlying disease process. The effect of this symptomatic suppression upon the long-term course of TD is unknown. GIMOTI is contraindicated in patients with a history of TD [see Contraindications (4)]. Avoid GIMOTI in patients receiving other drugs that are likely to cause TD (e.g., antipsychotics).
5.2 Other Extrapyramidal Symptoms
In addition to TD, metoclopramide may cause other extrapyramidal symptoms (EPS), parkinsonian symptoms, and motor restlessness. Advise patients to seek immediate medical attention if such symptoms occur and to discontinue GIMOTI.
- Extrapyramidal symptoms (EPS), such as acute dystonic reactions, occurred in patients treated with oral metoclopramide dosages of 30 mg to 40 mg daily. Such reactions occurred more frequently in adults less than 30 years of age and at higher than recommended dosages. EPS occurred more frequently in pediatric patients compared to adults (GIMOTI is not approved for use in pediatric patients). Symptoms can occur in the first 24 to 48 hours after starting metoclopramide. Symptoms included involuntary movements of limbs and facial grimacing, torticollis, oculogyric crisis, rhythmic protrusion of tongue, bulbar type of speech, trismus, or dystonic reactions resembling tetanus. Rarely, dystonic reactions were present as stridor and dyspnea, possibly due to laryngospasm. Diphenhydramine hydrochloride or benztropine mesylate may be used to treat these adverse reactions. Avoid GIMOTI in patients receiving other drugs that can cause EPS (e.g., antipsychotics).
- Parkinsonian symptoms (bradykinesia, tremor, cogwheel rigidity, mask-like facies) have occurred after starting metoclopramide, more commonly within the first 6 months, but also after longer periods. Symptoms generally have subsided within 2 to 3 months after discontinuation of metoclopramide. Avoid GIMOTI in patients with Parkinson's disease and other patients being treated with antiparkinsonian drugs due to potential exacerbation of symptoms. Avoid treatment with metoclopramide (all dosage forms and routes of administration) for more than 12 weeks [see Dosage and Administration (2.1), Warnings and Precautions (5.1)].
- Motor restlessness (akathisia) has developed and consisted of feelings of anxiety, agitation, jitteriness, and insomnia, as well as inability to sit still, pacing, and foot tapping. Discontinue GIMOTI if these symptoms develop.
5.3 Neuroleptic Malignant Syndrome
Metoclopramide may cause a potentially fatal symptom complex called neuroleptic malignant syndrome (NMS). NMS has been reported in association with metoclopramide overdosage and concomitant treatment with another drug associated with NMS. Avoid GIMOTI in patients receiving other drugs associated with NMS, including typical and atypical antipsychotics.
Clinical manifestations of NMS include hyperpyrexia, muscle rigidity, altered mental status, and manifestations of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac arrhythmias). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure. Patients with such symptoms should be evaluated immediately.
In the diagnostic evaluation, consider the presence of other serious medical conditions (e.g., pneumonia, systemic infection) and untreated or inadequately treated extrapyramidal signs and symptoms. Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, malignant hyperthermia, drug fever, serotonin syndrome, and primary central nervous system pathology.
Management of NMS includes:
- Immediate discontinuation of GIMOTI and other drugs not essential to concurrent therapy [see Drug Interactions (7.1)].
- Intensive symptomatic treatment and medical monitoring.
- Treatment of any concomitant serious medical problems for which specific treatments are available.
5.4 Depression
Depression has occurred in metoclopramide-treated patients with and without a history of depression. Symptoms have included suicidal ideation and suicide. Avoid GIMOTI use in patients with a history of depression.
5.5 Hypertension
Metoclopramide may elevate blood pressure. In one study in hypertensive patients, intravenously administered metoclopramide was shown to release catecholamines; hence, avoid GIMOTI use in patients with hypertension or in patients taking monoamine oxidase inhibitors [see Drug Interactions (7.1)].
There are also clinical reports of hypertensive crises in patients with undiagnosed pheochromocytoma. GIMOTI is contraindicated in patients with pheochromocytoma or other catecholamine-releasing paragangliomas [see Contraindications (4)]. Discontinue GIMOTI in any patient with a rapid rise in blood pressure.
5.6 Fluid Retention
Because metoclopramide produces a transient increase in plasma aldosterone, patients with cirrhosis or congestive heart failure may be at risk of developing fluid retention and volume overload. Discontinue GIMOTI if any of these adverse reactions occur.
5.7 Hyperprolactinemia
As with other dopamine D2 receptor antagonists, metoclopramide elevates prolactin levels. Hyperprolactinemia may suppress hypothalamic gonadotropin-releasing hormone, resulting in reduced pituitary gonadotropin secretion. This, in turn, may inhibit reproductive function by impairing gonadal steroidogenesis in both female and male patients. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported with prolactin-elevating drugs, including metoclopramide.
Hyperprolactinemia may potentially stimulate prolactin-dependent breast cancer. However, some clinical studies and epidemiology studies have not shown an association between administration of dopamine D2 receptor antagonists and tumorigenesis in humans [see Nonclinical Toxicology (13.1)].
5.8 Effects on the Ability to Drive and Operate Machinery
Metoclopramide may impair the mental and/or physical abilities required for the performance of hazardous tasks such as operating machinery or driving a motor vehicle. Concomitant use of central nervous system (CNS) depressants or drugs associated with EPS may increase this effect (e.g., alcohol, sedatives, hypnotics, opiates, and anxiolytics). Avoid GIMOTI or the interacting drug, depending on the importance of the drug to the patient [see Drug Interactions (7.1)].
5.9 Risk of Adverse Reactions with GIMOTI in Patients with Moderate or Severe Renal and Hepatic Impairment, CYP2D6 Poor Metabolizers and Patients Taking Strong CYP2D6 Inhibitors
Patients with moderate or severe renal or hepatic impairment, patients who are CYP2D6 poor metabolizers, and patients concurrently using strong CYP2D6 inhibitors have increased exposure to metoclopramide from GIMOTI due to reduced metabolism or excretion which may lead to an increased risk of adverse reactions, including tardive dyskinesia. Use of GIMOTI is not recommended in these patient populations since the dose of GIMOTI cannot be adjusted to reduce exposure [see Drug Interactions (7.1), Use in Specific Populations (8.6, 8.7, 8.9)].
6. Adverse Reactions/Side Effects
The following adverse reactions are described, or described in greater detail, in other sections of the labeling:
- Tardive dyskinesia [see Boxed Warning and Warnings and Precautions (5.1)]
- Other extrapyramidal effects [see Warnings and Precautions (5.2)]
- Neuroleptic malignant syndrome [see Warnings and Precautions (5.3)]
- Depression [see Warnings and Precautions (5.4)]
- Hypertension [see Warnings and Precautions (5.5)]
- Fluid retention [see Warnings and Precautions (5.6)]
- Hyperprolactinemia [see Warnings and Precautions (5.7)]
- Effects on the ability to drive and operate machinery [see Warnings and Precautions (5.8)]
The following adverse reactions have been identified from clinical studies or postmarketing reports of metoclopramide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The safety of GIMOTI was evaluated in clinical trials of patients with gastroparesis and established in clinical trials of oral metoclopramide.
7. Drug Interactions
7.1 Effects of Other Drugs on Metoclopramide
Table 1 displays the effects of other drugs on metoclopramide.
| Antipsychotics | |
| Clinical Impact | Potential for additive effects, including increased frequency and severity of tardive dyskinesia (TD), other extrapyramidal symptoms (EPS), and neuroleptic malignant syndrome (NMS). |
| Intervention | Avoid concomitant use [see Warnings and Precautions (5.1, 5.2, 5.3)]. |
| Strong CYP2D6 Inhibitors, not Included in Antipsychotic Category Above | |
| Clinical Impact | Increased plasma concentrations of metoclopramide; risk of exacerbation of extrapyramidal symptoms [see Clinical Pharmacology (12.3)]. |
| Intervention | Use of GIMOTI is not recommended [see Warnings and Precautions (5.9)]. |
| Examples | quinidine, bupropion, fluoxetine, and paroxetine |
| Monoamine Oxidase Inhibitors | |
| Clinical Impact | Increased risk of hypertension [see Warnings and Precautions (5.5)]. |
| Intervention | Avoid concomitant use. |
| Central Nervous System (CNS) Depressants | |
| Clinical Impact | Increased risk of CNS depression [see Warnings and Precautions (5.8)]. |
| Intervention | Avoid GIMOTI or the interacting drug, depending on the importance of the drug to the patient. |
| Examples | alcohol, sedatives, hypnotics, opiates, and anxiolytics |
| Drugs that Impair Gastrointestinal Motility | |
| Clinical Impact | Potential for decreased effect of metoclopramide due to opposing effects on gastrointestinal motility. |
| Intervention | Monitor for reduced effect on gastrointestinal motility. |
| Examples | antiperistaltic antidiarrheal drugs, anticholinergic drugs, and opiates |
| Dopaminergic Agonists and Other Drugs that Increase Dopamine Concentrations | |
| Clinical Impact | Decreased therapeutic effect of metoclopramide due to opposing effects on dopamine. |
| Intervention | Monitor for reduced therapeutic effect. |
| Examples | apomorphine, bromocriptine, cabergoline, levodopa, pramipexole, ropinirole, and rotigotine |
7.2 Effects of Metoclopramide on Other Drugs
Table 2 displays the effects of metoclopramide on other drugs.
|
|
| Dopaminergic Agonists and Drugs Increasing Dopamine Concentrations | |
| Clinical Impact | Opposing effects of metoclopramide and the interacting drug on dopamine. Potential exacerbation of symptoms (e.g., parkinsonian symptoms). |
| Intervention | Avoid concomitant use [see Warnings and Precautions (5.2)]. |
| Examples | Apomorphine, bromocriptine, cabergoline, levodopa, pramipexole, ropinirole, rotigotine |
| Succinylcholine, Mivacurium | |
| Clinical Impact | Metoclopramide inhibits plasma cholinesterase leading to enhanced neuromuscular blockade. |
| Intervention | Monitor for signs and symptoms of prolonged neuromuscular blockade |
| Drugs with Absorption Altered due to Increased Gastrointestinal Motility | |
| Clinical Impact | The effect of metoclopramide on other drugs is variable. Increased gastrointestinal (GI) motility by metoclopramide may impact absorption of other drugs leading to decreased or increased drug exposure. |
| Intervention | Drugs with Decreased Absorption (e.g., digoxin, atovaquone, posaconazole oral suspension*, fosfomycin): Monitor for reduced therapeutic effect of the interacting drug. For digoxin, monitor therapeutic drug concentrations and increase the digoxin dose as needed (see prescribing information for digoxin). Drugs with Increased Absorption (e.g., sirolimus, tacrolimus, cyclosporine): Monitor therapeutic drug concentrations and adjust the dose as needed. See prescribing information for the interacting drug. |
| Insulin | |
| Clinical Impact | Increased GI motility by metoclopramide may increase delivery of food to the intestines and increase blood glucose. |
| Intervention | Monitor blood glucose and adjust insulin dosage regimen as needed. |
8. Use In Specific Populations
8.2 Lactation
Clinical Considerations
Monitor breastfeeding neonates because metoclopramide may cause extrapyramidal signs (dystonias) and methemoglobinemia [see Warnings and Precautions (5.1, 5.2), Use in Specific Populations (8.4)].
Data
In published clinical studies, the estimated amount of metoclopramide received by the breastfed infant was less than 10% of the maternal weight-adjusted oral dose. In one study, the estimated daily amount of metoclopramide received by infants from breast milk ranged from 6 to 24 mcg/kg/day in early puerperium (3 to 9 days postpartum) and from 1 to 13 mcg/kg/day at 8 to 12 weeks postpartum.
8.4 Pediatric Use
Metoclopramide is not recommended for use in pediatric patients due to the risk of tardive dyskinesia (TD) and other extrapyramidal symptoms as well as the risk of methemoglobinemia in neonates. The safety and effectiveness of GIMOTI in pediatric patients have not been established.
Dystonias and other extrapyramidal symptoms associated with metoclopramide are more common in pediatric patients than in adults [see Indications and Usage (1), Warnings and Precautions (5.1, 5.2)]. In addition, neonates have reduced levels of NADH-cytochrome b5 reductase, making them more susceptible to methemoglobinemia, a possible adverse reaction of metoclopramide use in neonates [see Use in Specific Populations (8.8)].
8.5 Geriatric Use
Metoclopramide is known to be substantially excreted by the kidney, and the risk of adverse reactions, including tardive dyskinesia (TD), may be greater in patients with impaired renal function [see Warnings and Precautions (5.1), Use in Specific Populations (8.6), Clinical Pharmacology (12.3)]. Elderly patients are more likely to have decreased renal function and may be more sensitive to the adverse effects of metoclopramide, especially elderly women, and require a lower starting dosage. GIMOTI is not recommended in geriatric patients as initial therapy. Geriatric patients receiving an alternative metoclopramide product at a stable dosage of 10 mg four times daily can be switched to GIMOTI [see Dosage and Administration (2.2)].
8.6 Renal Impairment
The clearance of metoclopramide is decreased and the systemic exposure is increased in patients with moderate to severe renal impairment compared to patients with normal renal function, which may increase the risk of adverse reactions [see Clinical Pharmacology (12.3)]. GIMOTI is not recommended in patients with moderate and severe renal impairment (creatinine clearance less than 60 mL/minute), including those receiving hemodialysis and continuous ambulatory peritoneal dialysis [see Warnings and Precautions (5.9)]. Use the recommended dosage of GIMOTI in patients with mild renal impairment (creatinine clearance 60 mL/minute or greater) [see Dosage and Administration (2.2)].
8.7 Hepatic Impairment
Patients with severe hepatic impairment (Child-Pugh C) have reduced systemic metoclopramide clearance (by approximately 50%) compared to patients with normal hepatic function [see Clinical Pharmacology (12.3)]. The resulting increase in metoclopramide blood concentrations increases the risk of adverse reactions. There are no pharmacokinetic data in patients with moderate hepatic impairment (Child-Pugh B). GIMOTI is not recommended in patients with moderate or severe (Child-Pugh B or C) hepatic impairment [see Warnings and Precautions (5.9)]. Use the recommended dosage of GIMOTI in patients with mild hepatic impairment (Child-Pugh A) [see Dosage and Administration (2.2)].
Metoclopramide, by producing a transient increase in plasma aldosterone, may increase the risk of fluid retention in patients with hepatic impairment [see Warnings and Precautions (5.6)]. Monitor patients with hepatic impairment for the occurrence of fluid retention and volume overload.
8.8 NADH-Cytochrome b5 Reductase Deficiency
Metoclopramide-treated patients with NADH-cytochrome b5 reductase deficiency are at an increased risk of developing methemoglobinemia and/or sulfhemoglobinemia. For patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency with metoclopramide-induced methemoglobinemia, methylene blue treatment is not recommended. Methylene blue may cause hemolytic anemia in patients with G6PD deficiency, which may be fatal [see Overdosage (10)].
8.9 CYP2D6 Poor Metabolizers
Metoclopramide is a substrate of CYP2D6. The elimination of metoclopramide may be slowed in patients who are CYP2D6 poor metabolizers (compared to patients who are CYP2D6 intermediate, extensive, or ultra-rapid metabolizers), possibly increasing the risk of dystonic and other adverse reactions to metoclopramide [see Clinical Pharmacology (12.3)]. GIMOTI is not recommended in patients who are CYP2D6 poor metabolizers [see Warnings and Precautions (5.9)].
10. Overdosage
Manifestations of metoclopramide overdosage included drowsiness, disorientation, extrapyramidal reactions, other adverse reactions associated with metoclopramide use (including, e.g., methemoglobinemia), and sometimes death. Neuroleptic malignant syndrome (NMS) has been reported in association with metoclopramide overdose and concomitant treatment with another drug associated with NMS [see Warnings and Precautions (5.1, 5.2, 5.3)].
There are no specific antidotes for metoclopramide overdosage. If over-exposure occurs, call your Poison Control Center at 1-800-222-1222 for current information on the management of poisoning or overdosage.
Methemoglobinemia can be reversed by the intravenous administration of methylene blue. However, methylene blue may cause hemolytic anemia in patients with G6PD deficiency, which may be fatal.
Hemodialysis and continuous ambulatory peritoneal dialysis do not remove significant amounts of metoclopramide.
11. Gimoti Description
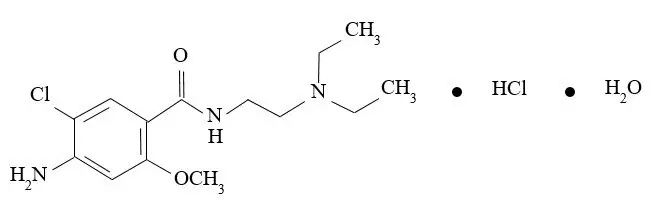
Metoclopramide hydrochloride, the active ingredient in GIMOTI, is a dopamine-2 receptor antagonist. Metoclopramide hydrochloride is a white, crystalline, odorless substance, freely soluble in water. Its chemical name is 4-amino5-chloro-N-[2-(diethylamino)ethyl]-2-methoxy benzamide monohydrochloride monohydrate.
The molecular formula is C14H22ClN3O2∙HCl∙H2O. Its molecular weight is 354.3. The structural formula is:
GIMOTI (metoclopramide) nasal spray is for nasal administration. The product is supplied as an aqueous solution with a pH of 5.5 ± 0.5 in a 10 mL amber glass vial fitted with a metered spray pump attachment. Each unit contains 9.8 mL.
Each 70 microliter spray contains 15 mg metoclopramide, equivalent to 17.73 mg of metoclopramide hydrochloride. Inactive ingredients consist of benzalkonium chloride, citric acid monohydrate, edetate disodium dihydrate, purified water, sodium citrate dihydrate, and sorbitol.
12. Gimoti - Clinical Pharmacology
12.1 Mechanism of Action
Metoclopramide stimulates motility of the upper gastrointestinal tract without stimulating gastric, biliary, or pancreatic secretions. The exact mechanism of action of metoclopramide in the treatment of gastroesophageal reflux and acute and recurrent diabetic gastroparesis has not been fully established. It seems to sensitize tissues to the action of acetylcholine. The effect of metoclopramide on motility is not dependent on intact vagal innervation, but it can be abolished by anticholinergic drugs.
Metoclopramide increases the tone and amplitude of gastric (especially antral) contractions, relaxes the pyloric sphincter and the duodenal bulb, and increases peristalsis of the duodenum and jejunum resulting in accelerated gastric emptying and intestinal transit. It increases the resting tone of the lower esophageal sphincter. It has little, if any, effect on the motility of the colon or gallbladder.
14. Clinical Studies
The effectiveness of GIMOTI has been established based on studies of oral metoclopramide for the relief of symptoms in adults with acute and recurrent diabetic gastroparesis.
16. How is Gimoti supplied
GIMOTI (metoclopramide) nasal spray is supplied as a solution of metoclopramide in a 10 mL Type 1 amber glass bottle fitted with a metered spray pump attachment, a protective cap, and a safety clip. Each box of GIMOTI (NDC 72089-307-15) contains 1 bottle, with FDA-approved Patient Labeling (see Instructions for Use for proper actuation of the device).
Each actuation delivers 15 mg of metoclopramide. Each bottle contains 9.8 mL which is sufficient for 4 weeks of 4 times a day use.
17. Patient Counseling Information
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
| This Medication Guide has been approved by the U.S. Food and Drug Administration | Approved: June 2020 |
| Medication Guide
GIMOTI™ (jye-MOH-tee) (metoclopramide) nasal spray |
|
| Read this Medication Guide before you start taking GIMOTI and each time you get a refill. There may be new information. If you take another product that contains metoclopramide (such as REGLAN tablets, REGLAN injection, metoclopramide orally disintegrating tablets [ODT], or metoclopramide oral solution), you should read the Medication Guide that comes with that product. Some of the information may be different. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment. | |
| What is the most important information I should know about GIMOTI?
GIMOTI can cause serious side effects, including: Tardive dyskinesia (abnormal muscle movements). These movements happen mostly in the face muscles. You cannot control these movements. They may not go away even after stopping GIMOTI. Your chances for getting tardive dyskinesia increase:
Call your healthcare provider right away if you get movements you cannot stop or control, such as:
See the section "What are the possible side effects of GIMOTI?" for more information about side effects. |
|
What is GIMOTI?
|
|
Do not take GIMOTI if you:
|
|
Before taking GIMOTI, tell your healthcare provider about all of your medical conditions, including if you:
GIMOTI may affect the way other medicines work and other medicines may affect how GIMOTI works. Tell your healthcare provider before you start or stop other medicines. Especially tell your healthcare provider if you take:
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
|
How should I take GIMOTI?
|
|
What should I avoid while taking GIMOTI?
|
|
What are the possible side effects of GIMOTI? GIMOTI can cause serious side effects, including:
You may still have side effects after stopping GIMOTI. You may have symptoms from stopping GIMOTI such as headaches and feeling dizzy or nervous. Tell your healthcare provider about any side effect that bothers you or that does not go away. These are not all the possible side effects of GIMOTI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store GIMOTI?
|
|
| General information about the safe and effective use of GIMOTI.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use GIMOTI for a condition for which it was not prescribed. Do not give GIMOTI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about GIMOTI that is written for health professionals. |
|
| What are the ingredients in GIMOTI?
Active ingredient: metoclopramide Inactive ingredients: benzalkonium chloride, citric acid monohydrate, edetate disodium dihydrate, purified water, sodium citrate dihydrate, sorbitol. Manufactured for Evoke Pharma, Inc. by Patheon, a Division of Thermo Fisher. GIMOTI is a trademark of Evoke Pharma. ©2020 Evoke Pharma, Inc. All rights reserved. For more information, go to www.evokepharma.com or call 1-833-4-GIMOTI (1-833-444-6684). |
|
Instructions for Use
GIMOTI™ (jye-MOH-tee) (phonetic spelling)
(metoclopramide)
nasal spray
Read this Instructions for Use before you start using GIMOTI nasal spray and each time you get a refill. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Important information:
- GIMOTI is for use in your nose only.
- The GIMOTI nasal spray bottle has enough medicine for 4 weeks of treatment if taken 4 times each day.
- 1 dose is 1 spray in 1 nostril.
- Your GIMOTI nasal spray bottle must be primed:
- before you use it for the first time,
- when the nasal spray has not been used for 2 weeks.
- Store the GIMOTI nasal spray at room temperature between 68°F to 77°F (20°C to 25°C).
- Throw away (discard) the GIMOTI nasal spray 4 weeks after opening even if the bottle contains unused medicine.
- Keep out of the reach of children.
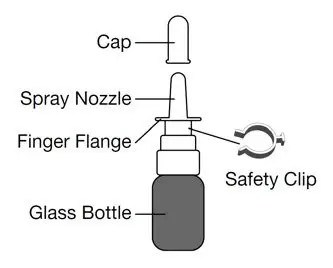
Parts of your GIMOTI bottle
Steps to use GIMOTI
- 1.
-
Uncap the GIMOTI nasal spray bottle
- Remove the cap from the spray nozzle by pulling straight up.
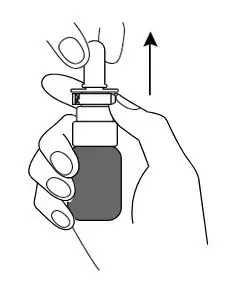
- Remove the cap from the spray nozzle by pulling straight up.
- 2.
-
Prime the GIMOTI nasal spray bottle
- Remove the safety clip from the spray pump.
- Place your index finger and middle finger on each side of the finger flange and your thumb on the bottom of the glass bottle.
- Turn the spray nozzle away from your face, keeping the bottle upright. Do not spray into your eyes.
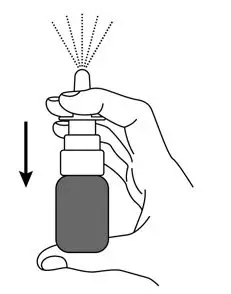
- Press down firmly and release 10 times on the finger flange until a spray appears from the spray nozzle. You may not see a spray the first few times you press down. After pressing down and releasing a spray 10 times, the GIMOTI nasal spray will be primed and ready to use.
If you are not able to press and release 10 sprays from the GIMOTI nasal spray, call your healthcare provider or pharmacist.
The GIMOTI nasal spray bottle is now ready for use.
- 3.
-
Using the GIMOTI nasal spray
- Place the spray nozzle tip under one of your nostrils. Tilt your head slightly forward so the tip of the spray nozzle is aimed away from the center of your nose.
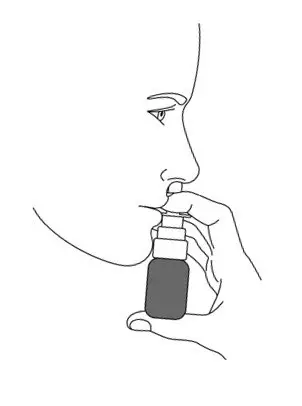
- Close the other nostril with your other index finger. Move the spray pump up so the tip of the nozzle is in your nostril.
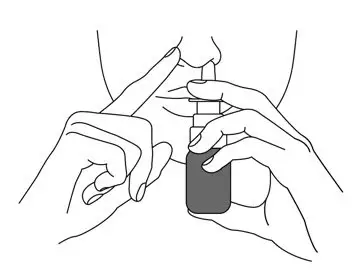
- Press down firmly on the finger flange until it stops moving to deliver a full dose.
- Release the finger flange and breathe in gently through the open nostril.
- Remove the spray pump nozzle tip from your nostril and breathe out slowly through your mouth.
- After use, wipe the spray nozzle with a clean tissue. Place the cap on the spray nozzle by pushing straight down. Place the safety clip back onto the spray pump.
- Place the spray nozzle tip under one of your nostrils. Tilt your head slightly forward so the tip of the spray nozzle is aimed away from the center of your nose.
Cleaning the spray pump nozzle
If the spray pump nozzle becomes clogged, remove it for cleaning by grasping the base of the spray nozzle and pulling up.
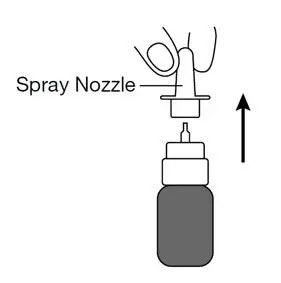
Soak the spray nozzle in warm water and rinse. Do not try to unblock the spray nozzle by inserting a pin or other sharp object because this will damage the spray nozzle.
Dry the spray nozzle at room temperature. When the spray nozzle is dry, place the dry spray nozzle back on the GIMOTI bottle.
Disposal instructions
The used GIMOTI nasal spray may be thrown away (discarded) in the household trash.
Manufactured for Evoke Pharma, Inc. by Patheon, a Division of Thermo Fisher.
GIMOTI is a trademark of Evoke Pharma. ©2020 Evoke Pharma, Inc. All rights reserved.
For more information, go to www.evokepharma.com or call 1-833-4-GIMOTI (1-833-444-6684).
These Instructions for Use have been approved by the U.S. Food and Drug Administration
Approved: June 2020
| GIMOTI
metoclopramide hydrochloride spray |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Evoke Pharma, Inc. (806873878) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon France SAS | 543127229 | MANUFACTURE(72089-307) , LABEL(72089-307) , PACK(72089-307) , ANALYSIS(72089-307) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| COSMA S.p.A. | 428655732 | API MANUFACTURE(72089-307) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Intertek | 503337305 | ANALYSIS(72089-307) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SGS France | 270520689 | ANALYSIS(72089-307) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ACM Pharma | 384719527 | ANALYSIS(72089-307) | |