Drug Detail:Mirapex (Pramipexole [ pram-i-pex-ole ])
Drug Class: Dopaminergic antiparkinsonism agents
Highlights of Prescribing Information
MIRAPEX safely and effectively. See full prescribing information for MIRAPEX.
MIRAPEX® (pramipexole dihydrochloride) tablets, for oral use
Initial U.S. Approval: 1997
Recent Major Changes
Indications and Usage for Mirapex
MIRAPEX is a non-ergot dopamine agonist indicated for the treatment of:
- Parkinson’s disease (PD) (1.1)
- Moderate-to-severe primary Restless Legs Syndrome (RLS) (1.2)
Mirapex Dosage and Administration
| * Doses should not be increased more frequently than every 5-7 days. Titrate to effective dose. If used with levodopa, may need to reduce levodopa dose. | ||
| Parkinson’s Disease-Normal Renal Function* (2.2) | ||
| Week | Dosage (mg) | Total Daily Dose (mg) |
| 1 | 0.125 TID | 0.375 |
| 2 | 0.25 TID | 0.75 |
| 3 | 0.5 TID | 1.5 |
| 4 | 0.75 TID | 2.25 |
| 5 | 1 TID | 3 |
| 6 | 1.25 TID | 3.75 |
| 7 | 1.5 TID | 4.5 |
| Parkinson’s Disease-Impaired Renal Function (2.2) | ||
| Creatinine Clearance | Starting Dose (mg) | Maximum Dose (mg) |
| >50 mL/min | 0.125 TID | 1.5 TID |
| 30 to 50 mL/min | 0.125 BID | 0.75 TID |
| 15 to 30 mL/min | 0.125 QD | 1.5 QD |
| <15 mL/min and hemodialysis patients | Data not available | |
| * Dosing interval is 4-7 days (14 days in patients with CrCl 20-60 mL/min) | |
| Restless Legs Syndrome* (2.3) | |
| Titration Step | Dose (mg) 2-3 hours before bedtime |
| 1 | 0.125 |
| 2 (if needed) | 0.25 |
| 3 (if needed) | 0.5 |
Dosage Forms and Strengths
Tablets: 0.125 mg, 0.25 mg, 0.5 mg, 0.75 mg, 1 mg, and 1.5 mg (3)
Contraindications
None (4)
Warnings and Precautions
- Falling Asleep During Activities of Daily Living: Sudden onset of sleep may occur without warning; advise patients to report symptoms (5.1)
- Symptomatic Orthostatic Hypotension: Monitor during dose escalation (5.2)
- Impulse Control/Compulsive Behaviors: Patients may experience compulsive behaviors and other intense urges (5.3)
- Hallucinations and Psychotic-like Behavior: May occur; risk increases with age (5.4)
- Dyskinesia: May be caused or exacerbated by MIRAPEX (5.5)
- Postural Deformity: Consider reducing the dose or discontinuing MIRAPEX if postural deformity occurs (5.6)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence >5% and greater than placebo):
- Early PD without levodopa: nausea, dizziness, somnolence, insomnia, constipation, asthenia, and hallucinations (6.1)
- Advanced PD with levodopa: postural (orthostatic) hypotension, dyskinesia, extrapyramidal syndrome, insomnia, dizziness, hallucinations, accidental injury, dream abnormalities, confusion, constipation, asthenia, somnolence, dystonia, gait abnormality, hypertonia, dry mouth, amnesia, and urinary frequency (6.1)
- RLS: nausea, somnolence, fatigue, and headache (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Boehringer Ingelheim Pharmaceuticals, Inc. at (800) 542-6257 or (800) 459-9906 TTY or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Dopamine antagonists: May diminish the effectiveness of pramipexole (7.1)
Use In Specific Populations
Pregnancy: Based on animal data, may cause fetal harm (8.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2020
Full Prescribing Information
2. Mirapex Dosage and Administration
2.2 Dosing for Parkinson's Disease
| Week | Dosage (mg) | Total Daily Dose (mg) |
| 1 | 0.125 three times a day | 0.375 |
| 2 | 0.25 three times a day | 0.75 |
| 3 | 0.5 three times a day | 1.50 |
| 4 | 0.75 three times a day | 2.25 |
| 5 | 1 three times a day | 3.0 |
| 6 | 1.25 three times a day | 3.75 |
| 7 | 1.5 three times a day | 4.50 |
| Renal Status | Starting Dose (mg) | Maximum Dose (mg) |
| Normal to mild
impairment (creatinine Cl >50 mL/min) | 0.125 three times a day | 1.5 three times a day |
| Moderate impairment (creatinine Cl =30 to 50 mL/min) | 0.125 twice a day | 0.75 three times a day |
| Severe impairment (creatinine Cl =15 to <30 mL/min) | 0.125 once a day | 1.5 once a day |
| Very severe impairment (creatinine Cl <15 mL/min and hemodialysis patients) | The use of MIRAPEX tablets has not been adequately studied in this group of patients. | |
3. Dosage Forms and Strengths
- 0.125 mg: white, round tablet with “BI” on one side and “83” on the reverse side. Each tablet contains 0.125 mg pramipexole dihydrochloride monohydrate equivalent to 0.118 mg pramipexole dihydrochloride.
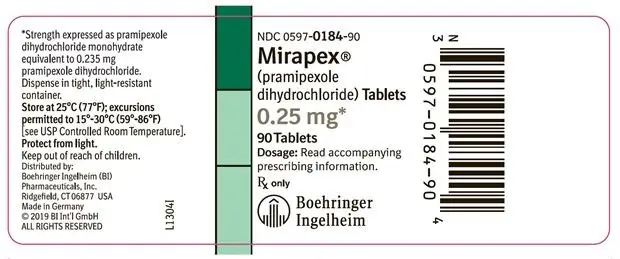
- 0.25 mg: white, oval, scored tablet with “BI BI” on one side and “84 84” on the reverse side. Each tablet contains 0.25 mg pramipexole dihydrochloride monohydrate equivalent to 0.235 mg pramipexole dihydrochloride.
- 0.5 mg: white, oval, scored tablet with “BI BI” on one side and “85 85” on the reverse side. Each tablet contains 0.5 mg pramipexole dihydrochloride monohydrate equivalent to 0.47 mg pramipexole dihydrochloride.
- 0.75 mg: white, oval, debossed tablet with “BI” on one side and “101” on the reverse side. Each tablet contains 0.75 mg pramipexole dihydrochloride monohydrate equivalent to 0.705 mg pramipexole dihydrochloride.
- 1 mg: white, round, scored tablet with “BI BI” on one side and “90 90” on the reverse side. Each tablet contains 1 mg pramipexole dihydrochloride monohydrate equivalent to 0.94 mg pramipexole dihydrochloride.
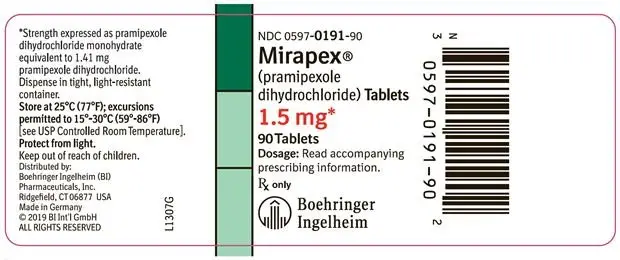
- 1.5 mg: white, round, scored tablet with “BI BI” on one side and “91 91” on the reverse side. Each tablet contains 1.5 mg pramipexole dihydrochloride monohydrate equivalent to 1.41 mg pramipexole dihydrochloride.
5. Warnings and Precautions
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Falling Asleep During Activities of Daily Living and Somnolence [see Warnings and Precautions (5.1)].
- Symptomatic Orthostatic Hypotension [see Warnings and Precautions (5.2)].
- Impulse Control/Compulsive Behaviors [see Warnings and Precautions (5.3)].
- Hallucinations and Psychotic-like Behavior [see Warnings and Precautions (5.4)].
- Dyskinesia [see Warnings and Precautions (5.5)].
- Postural Deformity [see Warnings and Precautions (5.6)].
- Rhabdomyolysis [see Warnings and Precautions (5.8)].
- Retinal Pathology [see Warnings and Precautions (5.9)].
- Events Reported with Dopaminergic Therapy [see Warnings and Precautions (5.10)].
6.1 Clinical Trials Experience
| Body System/Adverse Reaction | MIRAPEX (N=388) % | Placebo (N=235) % |
| Nervous System | ||
| Dizziness | 25 | 24 |
| Somnolence | 22 | 9 |
| Insomnia | 17 | 12 |
| Hallucinations | 9 | 3 |
| Confusion | 4 | 1 |
| Amnesia | 4 | 2 |
| Hypesthesia | 3 | 1 |
| Dystonia | 2 | 1 |
| Akathisia | 2 | 0 |
| Thinking abnormalities | 2 | 0 |
| Decreased libido | 1 | 0 |
| Myoclonus | 1 | 0 |
| Digestive System | ||
| Nausea | 28 | 18 |
| Constipation | 14 | 6 |
| Anorexia | 4 | 2 |
| Dysphagia | 2 | 0 |
| Body as a Whole | ||
| Asthenia | 14 | 12 |
| General edema | 5 | 3 |
| Malaise | 2 | 1 |
| Reaction unevaluable | 2 | 1 |
| Fever | 1 | 0 |
| Metabolic & Nutritional System | ||
| Peripheral edema | 5 | 4 |
| Decreased weight | 2 | 0 |
| Special Senses | ||
| Vision abnormalities | 3 | 0 |
| Urogenital System | ||
| Impotence | 2 | 1 |
| Body System/Adverse Reaction | MIRAPEX (N=260) % | Placebo (N=264) % |
| Nervous System | ||
| Dyskinesia | 47 | 31 |
| Extrapyramidal syndrome | 28 | 26 |
| Insomnia | 27 | 22 |
| Dizziness | 26 | 25 |
| Hallucinations | 17 | 4 |
| Dream abnormalities | 11 | 10 |
| Confusion | 10 | 7 |
| Somnolence | 9 | 6 |
| Dystonia | 8 | 7 |
| Gait abnormalities | 7 | 5 |
| Hypertonia | 7 | 6 |
| Amnesia | 6 | 4 |
| Akathisia | 3 | 2 |
| Thinking abnormalities | 3 | 2 |
| Paranoid reaction | 2 | 0 |
| Delusions | 1 | 0 |
| Sleep disorders | 1 | 0 |
| Cardiovascular System | ||
| Postural hypotension | 53 | 48 |
| Body as a Whole | ||
| Accidental injury | 17 | 15 |
| Asthenia | 10 | 8 |
| General edema | 4 | 3 |
| Chest pain | 3 | 2 |
| Malaise | 3 | 2 |
| Digestive System | ||
| Constipation | 10 | 9 |
| Dry mouth | 7 | 3 |
| Urogenital System | ||
| Urinary frequency | 6 | 3 |
| Urinary tract infection | 4 | 3 |
| Urinary incontinence | 2 | 1 |
| Respiratory System | ||
| Dyspnea | 4 | 3 |
| Rhinitis | 3 | 1 |
| Pneumonia | 2 | 0 |
| Special Senses | ||
| Accommodation abnormalities | 4 | 2 |
| Vision abnormalities | 3 | 1 |
| Diplopia | 1 | 0 |
| Musculoskeletal System | ||
| Arthritis | 3 | 1 |
| Twitching | 2 | 0 |
| Bursitis | 2 | 0 |
| Myasthenia | 1 | 0 |
| Metabolic & Nutritional System | ||
| Peripheral edema | 2 | 1 |
| Increased creatine PK | 1 | 0 |
| Skin & Appendages | ||
| Skin disorders | 2 | 1 |
| Body System/Adverse Reaction | MIRAPEX 0.125 – 0.75 mg/day (N=575) % | Placebo (N=223) % |
| Gastrointestinal disorders | ||
| Nausea | 16 | 5 |
| Constipation | 4 | 1 |
| Diarrhea | 3 | 1 |
| Dry mouth | 3 | 1 |
| Nervous system disorders | ||
| Headache | 16 | 15 |
| Somnolence | 6 | 3 |
| General disorders and administration site conditions | ||
| Fatigue | 9 | 7 |
| Infections and infestations | ||
| Influenza | 3 | 1 |
| Body System/Adverse Reaction | MIRAPEX 0.25 mg (N=88) % | MIRAPEX 0.5 mg (N=80) % | MIRAPEX 0.75 mg (N=90) % | Placebo (N=86) % |
| Gastrointestinal disorders | ||||
| Nausea | 11 | 19 | 27 | 5 |
| Diarrhea | 3 | 1 | 7 | 0 |
| Dyspepsia | 3 | 1 | 4 | 7 |
| Psychiatric disorders | ||||
| Insomnia | 9 | 9 | 13 | 9 |
| Abnormal dreams | 2 | 1 | 8 | 2 |
| General disorders and administration site conditions | ||||
| Fatigue | 3 | 5 | 7 | 5 |
| Musculoskeletal and connective tissue disorders | ||||
| Pain in extremity | 3 | 3 | 7 | 1 |
| Infections and infestations | ||||
| Influenza | 1 | 4 | 7 | 1 |
| Respiratory, thoracic and mediastinal disorders | ||||
| Nasal congestion | 0 | 3 | 6 | 1 |
8. Use In Specific Populations
8.4 Pediatric Use
Safety and effectiveness of MIRAPEX in pediatric patients has not been established.
12. Mirapex - Clinical Pharmacology
13. Nonclinical Toxicology
14. Clinical Studies
14.2 Restless Legs Syndrome
| *CGI-I responders = “much improved” and “very much improved” | |||||
| MIRAPEX 0.25 mg | MIRAPEX 0.5 mg | MIRAPEX 0.75 mg | MIRAPEX Total | Placebo | |
| No. Patients | 88 | 79 | 87 | 254 | 85 |
| IRLS score | -13.1 | -13.4 | -14.4 | -13.6 | -9.4 |
| CGI-I responders* | 74.7% | 67.9% | 72.9% | 72.0% | 51.2% |
| *CGI-I responders = “much improved” and “very much improved” | ||||||
| MIRAPEX 0.125 mg | MIRAPEX 0.25 mg | MIRAPEX 0.5 mg | MIRAPEX 0.75 mg | MIRAPEX Total | Placebo | |
| No. Patients | 21 | 22 | 22 | 21 | 86 | 21 |
| IRLS score | -11.7 | -15.3 | -17.6 | -15.2 | -15.0 | -6.2 |
| CGI-I responders* | 61.9% | 68.2% | 86.4% | 85.7% | 75.6% | 42.9% |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Impulse Control Symptoms Including Compulsive Behaviors
Hallucinations and Psychotic-like Behavior
Postural (Orthostatic) Hypotension
Distributed by:
Boehringer Ingelheim Pharmaceuticals,
Inc.
Ridgefield, CT 06877 USA
Licensed from:
Boehringer Ingelheim International
GmbH
Address medical inquiries to: (800) 542-6257 or (800) 459-9906 TTY.
Trademark under license from:
Boehringer
Ingelheim International GmbH
Copyright © 2020 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED
PATIENT INFORMATION
MIRAPEX® (mîr′-ah-pĕx)
(pramipexole dihydrochloride)
tablets
MIRAPEX is a prescription medicine used to treat:
- signs and symptoms of Parkinson's disease (PD)
- moderate to severe primary Restless Legs Syndrome (RLS)
It is not known if MIRAPEX is safe and effective in children.
What should I tell my doctor before taking MIRAPEX?
-
Before taking MIRAPEX, tell your doctor if you:
- feel sleepy during the day from a sleep problem other than Restless Legs Syndrome
- have low blood pressure, or if you feel dizzy or faint, especially when getting up from sitting or lying down
- have trouble controlling your muscles (dyskinesia)
- have kidney problems
- drink alcohol. Alcohol can increase the chance that MIRAPEX will make you feel sleepy or fall asleep when you should be awake.
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if MIRAPEX will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if MIRAPEX passes into your breast milk. You and your doctor should decide if you will take MIRAPEX or breastfeed. You should not do both.
Especially tell your doctor if you take:
- medicines called neuroleptics (phenothiazines, butyrophenones, thioxanthenes) or metoclopramide. MIRAPEX may not work as well if you take these medicines.
- extended-release pramipexole (MIRAPEX ER). Pramipexole is the active ingredient in both MIRAPEX and MIRAPEX ER. If you are taking MIRAPEX ER, you should not take MIRAPEX.
- any other medicines that make you sleepy or may increase the effects of MIRAPEX, such as cimetidine (Tagamet).
Ask your doctor for a list of these medicines if you are not sure.
- Take MIRAPEX exactly as your doctor tells you to take it.
- Your doctor will tell you how much MIRAPEX to take and when to take it. Do not take more or less MIRAPEX than your doctor tells you to.
- Your doctor may change your dose if needed.
- MIRAPEX can be taken with or without food. Taking MIRAPEX tablets with food may lower your chances of getting nausea.
- If you take more MIRAPEX than your doctor recommends, call your doctor or go to the nearest hospital emergency room right away.
- If you miss a dose, do not double your next dose. Skip the dose you missed and take your next regular dose.
- If you have Parkinson’s disease and your doctor tells you
to stop taking MIRAPEX, you should stop MIRAPEX slowly as directed
by your doctor. If you stop MIRAPEX too quickly you may have withdrawal
symptoms such as:
- fever
- confusion
- severe muscle stiffness
Do not stop taking MIRAPEX without talking to your doctor.
What should I avoid while taking MIRAPEX?
- Do not drink alcohol while taking MIRAPEX. It can increase your chance of having serious side effects. See “What are the possible side effects of MIRAPEX?”
- Do not drive a car, operate a machine, or do other dangerous activities until you know how MIRAPEX affects you. Sleepiness caused by MIRAPEX can happen as late as 1 year after you start your treatment.
What are the possible side effects of MIRAPEX?
MIRAPEX may cause serious side effects, including:
-
falling asleep during normal daily activities. MIRAPEX may cause you to fall asleep while you are doing daily activities
such as driving, talking with other people, or eating.
- Some people taking the medicine in MIRAPEX have had car accidents because they fell asleep while driving.
- Some patients did not feel sleepy before they fell asleep while driving. You could fall asleep without any warning.
-
low blood pressure when you sit or stand up quickly. You may have:
- dizziness
- nausea
- fainting
- sweating
-
unusual urges. Some people who take certain
medicines to treat Parkinson’s disease or RLS, including MIRAPEX,
have reported problems, such as gambling, compulsive eating, compulsive
buying, and increased sex drive.
If you or your family members notice that you are developing unusual urges or behaviors, talk to your doctor. -
hallucinations and other psychotic-like behavior (seeing visions, hearing sounds or feeling sensations that are not
real, confusion, excessive suspicion, aggressive behavior, agitation,
delusional beliefs and disorganized thinking). The chances of having
hallucinations or other psychotic-like changes are higher in people
taking MIRAPEX for Parkinson’s disease who are elderly (age 65 or
older).
If you have hallucinations or other psychotic-like changes, talk with your doctor right away. -
uncontrolled sudden movements (dyskinesia).
If you have new dyskinesia or your existing dyskinesia gets worse tell your doctor. - posture changes. Talk with your doctor if you have posture changes you cannot control. These may include your neck bending forward, bending forward at the waist, or tilting sideways when you sit, stand, or walk.
The most common side effects in people taking MIRAPEX for Parkinson’s disease are:
- nausea
- dizziness
- insomnia
- constipation
- muscle weakness
- abnormal dreams
- confusion
- memory problems (amnesia)
- urinating more often than normal
- Store MIRAPEX at room temperature from 68ºF to 77ºF (20ºC to 25ºC).
- Keep MIRAPEX out of the light.
- Keep MIRAPEX and all medicines out of the reach of children.
General Information about the safe and effective use of MIRAPEX.

What are the ingredients in MIRAPEX?
This Patient Information has been approved by the U.S. Food and Drug Administration.
Distributed by:
Boehringer Ingelheim Pharmaceuticals,
Inc.
Ridgefield, CT 06877 USA
Licensed from:
Boehringer Ingelheim International
GmbH
Trademark under license
from:
Boehringer Ingelheim International GmbH
Copyright © 2020 Boehringer Ingelheim International
GmbH
ALL RIGHTS RESERVED
| MIRAPEX
pramipexole dihydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| MIRAPEX
pramipexole dihydrochloride tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| MIRAPEX
pramipexole dihydrochloride tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| MIRAPEX
pramipexole dihydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| MIRAPEX
pramipexole dihydrochloride tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| MIRAPEX
pramipexole dihydrochloride tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Registrant - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| West-Ward Columbus Inc. | 058839929 | ANALYSIS(0597-0184, 0597-0190, 0597-0101, 0597-0185, 0597-0183, 0597-0191) , PACK(0597-0184, 0597-0185, 0597-0190, 0597-0191, 0597-0183, 0597-0101) , LABEL(0597-0191, 0597-0101, 0597-0184, 0597-0185, 0597-0183, 0597-0190) , MANUFACTURE(0597-0191, 0597-0184, 0597-0185, 0597-0183, 0597-0101, 0597-0190) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Pharma GmbH and Co. KG | 551147440 | ANALYSIS(0597-0184, 0597-0190, 0597-0101, 0597-0185, 0597-0183, 0597-0191) , API MANUFACTURE(0597-0185, 0597-0190, 0597-0191, 0597-0101, 0597-0183, 0597-0184) | |