Drug Detail:Oraqix (Lidocaine & prilocaine periodontal gel 2.5%/2.5%)
Drug Class: Topical anesthetics
Highlights of Prescribing Information
ORAQIX® (lidocaine and prilocaine periodontal gel) 2.5%/2.5% Initial U.S. Approval: 2003
Recent Major Changes
Warnings and Precautions, Methemoglobinemia (5.1)
Indications and Usage for Oraqix
Oraqix is an amide local anesthetic indicated for adults who require localized anesthesia in periodontal pockets during scaling and/or root planing. (1)
Oraqix Dosage and Administration
- Use the included blunt-tipped applicator and the dispenser, which is sold separately, to apply gel to gingival margins and periodontal pockets. (2)
- May be reapplied as needed up to a maximum of 5 cartridges per treatment session.(2)
Dosage Forms and Strengths
Periodontal gel containing: lidocaine 25 mg/mL and prilocaine 25 mg/mL. (3)
Contraindications
- A history of hypersensitivity to local anesthetics of the amide type (4)
- A history of hypersensitivity to any component of the product (4)
Warnings and Precautions
- Methemoglobinemia: Cases of methemoglobinemia have been reported in association with local anesthetic use. (5.1)
- DO NOT INJECT (5.2)
- Allergic and anaphylactic reactions can occur (5.3)
- Avoid contact with eyes (5.4)
- Use with caution in patients with severe hepatic disease (5.6)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence >15%) are application site reactions including pain, soreness, irritation, numbness, ulcerations, vesicles, edema, abscess and/or redness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact DENTSPLY Pharmaceutical at 1-800-989- 8826 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Drug Interactions
- Other local anesthetics or agents structurally related to local anesthetics (7.1)
- Drugs That May Cause Methemoglobinemia When Used With ORAQIX (7.2)
Use In Specific Populations
- Pregnancy: There have been no adequate and well-controlled studies in pregnant women. Oraqix should be used during pregnancy only if the benefits outweigh the risks. (8.1)
- Nursing Mothers: Exercise caution when administering to a nursing woman. (8.3)
- Pediatric Use: Safety and effectiveness in pediatric patients have not been established. (8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2018
Full Prescribing Information
1. Indications and Usage for Oraqix
Oraqix is an amide local anesthetic indicated for adults who require localized anesthesia in periodontal pockets during scaling and/or root planing.
2. Oraqix Dosage and Administration
2.1 General Dosing Information
DO NOT INJECT [see Warnings and Precautions (5.2)]
Apply Oraqix on the gingival margin around the selected teeth using the blunt-tipped applicator included in the package and the dispenser, which is sold separately. Wait 30 seconds, and then fill the periodontal pockets with Oraqix using the blunt-tipped applicator until the gel becomes visible at the gingival margin. Wait another 30 seconds before starting treatment. A longer waiting time does not enhance the anesthesia. Anesthetic effect, as assessed by probing of pocket depths, has a duration of approximately 20 minutes (individual overall range 14–31 minutes). If the anesthesia starts to wear off, Oraqix may be re-applied if needed. Typically, one cartridge (1.7g) or less of Oraqix will be sufficient for one quadrant of the dentition.
When administered, Oraqix should be a liquid. If it has formed a gel, it should be placed in a refrigerator (do not freeze) until it becomes a liquid again. When in the liquid state, the air bubble visible in the cartridge will move if the cartridge is tilted.
3. Dosage Forms and Strengths
Periodontal gel containing: lidocaine 25 mg/mL and prilocaine 25 mg/mL. Each cartridge contains 1.7 mL (42.5 mg of lidocaine and 42.5 mg of prilocaine).
4. Contraindications
Oraqix is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type or to any other component of the product.
5. Warnings and Precautions
5.1 Methemoglobinemia
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure and are characterized by a cyanotic skin discoloration and/or abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious central nervous system and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue ORAQIX and any other oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
5.2 DO NOT INJECT
Oraqix should not be used with standard dental syringes. Only use this product with the Oraqix blunt-tipped applicator and the dispenser which is available from DENTSPLY Pharmaceutical.
5.3 Allergic/anaphylactic reactions
Allergic and anaphylactic reactions associated with lidocaine or prilocaine in Oraqix can occur. These reactions may be characterized by urticaria, angioedema, bronchospasm, and shock. If these reactions occur they should be managed by conventional means.
5.4 Avoid Contact with Eyes
Oraqix coming in contact with the eye should be avoided because animal studies have demonstrated severe eye irritation. A loss of protective reflexes may allow corneal irritation and potential abrasion. If eye contact occurs, immediately rinse the eye with water or saline and protect it until normal sensation returns. In addition, the patient should be evaluated by an ophthalmologist, as indicated.
5.5 History of Drug Sensitivity
Patients allergic to paraminobenzoic acid derivatives (procaine, tetracaine, benzocaine, etc.) have not shown cross sensitivity to lidocaine and/or prilocaine. However, Oraqix should be used with caution in patients with a history of drug sensitivities, especially if the etiologic agent is uncertain.
6. Adverse Reactions/Side Effects
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug can not be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Although no major differences in adverse events between Oraqix and placebo-treated subjects were observed, all patients in the placebo-controlled studies received either Oraqix or a placebo gel (consisting of the vehicle in Oraqix without lidocaine or prilocaine). Therefore, it is not possible to determine if adverse events in each treatment group were attributable to the inactive ingredients comprising the Oraqix or vehicle or if adverse event rates were higher than expected background rates. Therefore, a causal relationship between the reported adverse reactions and Oraqix could neither be established nor ruled out.
Following SRP treatment with Oraqix in 391 patients, the most frequent adverse events were local reactions in the oral cavity (see following table). These events, which occurred in approximately 15% of patients, included pain, soreness, irritation, numbness, vesicles, ulcerations, edema and/or redness in the treated area. Of the 391 patients treated with Oraqix, five developed ulcerative lesions and two developed vesicles of mild to moderate severity near the site of SRP. In addition, ulcerative lesions in or near the treated area were also reported for three out of 168 patients who received placebo. Other symptoms reported in more than one patient were headache, taste perversion, nausea, fatigue, flu, respiratory infection, musculoskeletal pain and accident/injury.
| Each patient is counted only once per adverse event. The occurrence in a single patient is included in this table if the same symptom has been seen in at least one patient in another group. | |||
|---|---|---|---|
| System Organ Class PreferredTerm | Oraqix gel*
(N=391)n (%) | Placebo gel*
(N=168)n (%) | Lidocaine injection*
(N=170)n (%) |
|
|||
| Muscular-Skeletal System Disorders
Myalgia | 1(0) | 2(1) | |
| Arthralgia and/or Arthropathy | 1(0) | 1(1) | |
| Central & Peripheral Nervous System
Disorders | |||
| Headache | 8(2) | 3(2) | 5(3) |
| Dizziness | 1(0) | 1(1) | 1(1) |
| Special Senses Other, Disorders
Taste Perversion† | 8(2) | 1(1) | |
| Gastro-Intestinal System Disorders
Nausea | 3(1) | 1(1) | |
| Respiratory System Disorders
Respiratory Infection | 2(1) | 1(1) | |
| Rhinitis | 2(1) | ||
| Body as a whole-General Disorders
Accident and/or Injury | 2(1) | 2(1) | |
| Fatigue | 3(1) | 2(1) | |
| Flu-Like Disorder | 2(1) | ||
| Pain (remote from application site) | 1(0) | 1(1) | 1(1) |
| Application Site Disorders‡
Anesthesia Local | 2(1) | ||
| Application Site Reaction§ | 52(13) | 20(12) | |
7. Drug Interactions
7.1 Other Local Anesthetics or Agents Structurally Related to Local Anesthetics
Oraqix should be used with caution in combination with dental injection anesthesia, other local anesthetics, or agents structurally related to local anesthetics, e.g., Class 1 antiarrhythmics such as tocainide and mexiletine, as the toxic effects of these drugs are likely to be additive and potentially synergistic.
7.2 Drugs That May Cause Methemoglobinemia When Used With ORAQIX
Patients who are administered local anesthetics are at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics:
| Class | Examples |
|---|---|
| Nitrates/Nitrites | nitric oxide, nitroglycerin, nitroprusside, nitrous oxide |
| Local anesthetics | articaine, benzocaine, bupivacaine, lidocaine, mepivacaine, prilocaine, procaine, ropivacaine, tetracaine |
| Antineoplastic agents | cyclophosphamide, flutamide, hydroxyurea, isofamide, rasburicase |
| Antibiotics | dapsone, nitrofurantoin, para-aminosalicylic acid, sufonamides |
| Antimalarials | chloroquine, primaquine |
| Anticonvulsants | Phenobarbital, phenytoin, sodium valproate |
| Other drugs | acetaminophen, metoclopramide, quinine, sulfasalazine |
8. Use In Specific Populations
8.3 Nursing Mothers
Lidocaine and, possibly, prilocaine are excreted in breast milk. Caution should be exercised when Oraqix is administered to nursing women.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Very young children are more susceptible to methemoglobinemia. There have been reports of clinically significant methemoglobinemia in infants and children following excessive applications of lidocaine 2.5% topical cream [See Warnings and Precautions (5.1)].
8.5 Geriatric Use
Of the total number of subjects in clinical studies of Oraqix, 7% were aged 65 and over, while 1% were aged 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
10. Overdosage
10.1 Oraqix/Local anesthetic toxicity emergency
Oraqix used at the recommended doses is not likely to cause toxic plasma levels of lidocaine or prilocaine. However, if other local anesthetics are administered at the same time, e.g. topically or by injection, the toxic effects are thought to be additive and could result in an overdose with systemic toxic reactions. There is generally an increase in severity of symptoms with increasing plasma concentrations of lidocaine and/or prilocaine. Systemic CNS toxicity may occur over a range of plasma concentrations of local anesthetics. CNS toxicity may typically be found around 5000 ng/mL of lidocaine; however a small number of patients reportedly may show signs of toxicity at approximately 1000 ng/mL. Pharmacological thresholds for prilocaine are poorly defined. Central nervous system (CNS) symptoms usually precede cardiovascular manifestations. The plasma level of lidocaine observed after the maximum recommended dose (5 cartridges) of Oraqix in 11 patients exposed over 3 hours ranged from 157 to 552 ng/mL with a mean of 284 ng/mL ± 122 SD. The corresponding figure for prilocaine was 53-181 ng/mL with a mean of 106 ± 45 SD. [See Clinical Pharmacology (12.3)].
Systemic adverse effects of lidocaine and/or prilocaine are manifested by central nervous system and/or cardiovascular symptoms.
Clinical symptoms of systemic toxicity include CNS excitation and/or depression (light-headedness, hyperacusis, visual disturbances, muscular tremors, and general convulsions). Lidocaine and/or prilocaine may cause decreases in cardiac output, total peripheral resistance and mean arterial pressure. These changes may be attributable to direct depressant effects of these local anesthetic agents on the cardiovascular system. Cardiovascular manifestations may include hypotension, bradycardia, arrhythmia, and cardiovascular collapse.
11. Oraqix Description
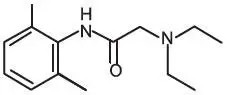
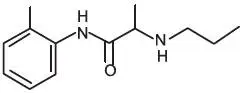
Oraqix (lidocaine and prilocaine periodontal gel,) 2.5%/2.5% is a microemulsion in which the oil phase is a eutectic mixture of lidocaine and prilocaine in a ratio of 1:1 by weight. This eutectic mixture has a melting point below room temperature; therefore, both local anesthetics exist as liquid oils rather than as crystals. Oraqix contains poloxamer excipients, which show reversible temperature-dependent gelation. Together with the lidocaine-prilocaine 1:1 mixture, the poloxamers form a low-viscosity fluid system at room temperature and an elastic gel in the periodontal pocket. Oraqix is administered into periodontal pockets, by means of the supplied special applicator. Gelation occurs at body temperature, followed by release of the local anesthetics, lidocaine and prilocaine. The Oraqix single-use glass cartridges deliver up to 1.7g (1.7mL) of gel (42.5 mg of lidocaine and 42.5 mg of prilocaine). Prilocaine base and lidocaine base are both relatively hydrophilic amino-amides.
The structural formulas are:
|
|
|
|
| Lidocaine C14H22N2O M.W. 234.3 | Prilocaine C13H20N2O M.W. 220.3 |
Lidocaine is chemically designated as 2-(diethylamino)-N-(2,6-dimethylphenyl)-acetamide and has an octanol:water partition ratio of 43 at pH 7.4. The pKa of lidocaine is 7.86. Prilocaine is chemically designated as N-(2-methyl-phenyl)-2 (propylamino)-propanamide and has an octanol:water partition ratio of 25 at pH 7.4. The pKa of prilocaine is 7.89. Each gram of Oraqix contains 25-mg lidocaine base and 25-mg prilocaine base. The gel also contains thermosetting agents (poloxamer 188 purified, poloxamer 407 purified), hydrochloric acid (pH adjustment), and purified water. The pH of Oraqix is 7.5-8.0.
12. Oraqix - Clinical Pharmacology
12.1 Mechanism of Action
Lidocaine and prilocaine belong to the amide class of local anesthetics. Both lidocaine and prilocaine block sodium ion channels required for the initiation and conduction of neuronal impulses, resulting in local anesthesia.
12.2 Pharmacodynamics
After application of Oraqix on the gingival margin and a waiting period of 30 seconds, additional Oraqix is applied directly into periodontal pockets to provide localized anesthesia. The onset of local anesthetic effect after application of Oraqix into the periodontal pocket occurs by 30 seconds and a longer waiting time does not enhance the anesthetic affect. Anesthetic effect, as assessed by probing of pocket depths, lasted for about 20 minutes (individual overall range 14 to 31 minutes).
14. Clinical Studies
A total of 337 patients (146 men and 191 women; 169 Oraqix and 168 placebo) were studied in three randomized, double-blind, placebo-controlled trials. Patients received a median dose of approximately 1 cartridge (1.7g gel), ranging from ¼ to 2½ cartridges per quadrant treated. The analgesic effect of Oraqix was assessed by asking patients to rate their pain on a continuous visual analog scale (VAS) from 0 (no pain) to 100 mm (worst pain imaginable). Patients were asked to report overall procedural pain 5 minutes following manual scaling and/or root planing (SRP) in a single quadrant that had been pre-treated with Oraqix or placebo (vehicle only, without lidocaine or prilocaine). In all three studies, patients were given Oraqix or placebo (vehicle only, without lidocaine or prilocaine). In all three studies, patients who were given Oraqix reported lower VAS scores during the procedure than those given placebo. Study B3 recruited patients with a known sensitivity to mechanical probing of dental pockets, whereas in studies B1 and B2, this was not a requirement. Results of B1, B2 and B3 are summarized below.
| Visual Analog Pain Scale | ||
|---|---|---|
| Study (No. of patients) | Oraqix Median VAS | Placebo Median VAS |
|
||
| B1 (n=122)* | 7 | 17 |
| B2 (n=130)* | 5 | 13 |
| B3 (n=85)* | 11 | 27 |
The trial also compared individual patient estimates of pain on a 5-step categorical Verbal Rating Scale (VRS) which included the following categories: no pain, mild pain, moderate pain, severe pain, and very severe pain. The results of those who reported no pain or mild pain are shown in the test table.
| Number of Patients Reporting "no pain" or "mild pain" during SRP | ||
|---|---|---|
| Study (No. of patients) | Oraqix Median VAS | Placebo Median VAS |
|
||
| B1 (n=122)* | 57 (90%) | 38 (64%) |
| B2 (n=130)* | 49 (78%) | 51 (76%) |
| B3 (n=85)* | 30 (70%) | 20 (48%) |
16. How is Oraqix supplied
Oraqix (lidocaine and prilocaine periodontal gel), 2.5%/2.5%, is supplied in dental cartridges that provide 1.7g gel. Individually blister-packaged cartridges of Oraqix are distributed in a carton of 20 (NDC 66312-110-20). Each individual blister package also contains a sterile blunt-tipped applicator. Each blunt-tipped applicator is for single use only. Oraqix is intended for use with the Oraqix Dispenser.
17. Patient Counseling Information
Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.
Patients should be cautioned to avoid injury to the treated area, or exposure to extreme hot or cold temperatures, until complete sensation has returned.
| ORAQIX
lidocaine and prilocaine gel |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Dentsply Pharmaceutical (102221942) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Recipharm Karlskoga | 351250415 | MANUFACTURE(66312-110) | |