Drug Detail:Provayblue (injection) (Methylene blue (injection) [ meth-i-leen-bloo ])
Drug Class: Antidotes Miscellaneous diagnostic dyes
Highlights of Prescribing Information
PROVAYBLUE ® (methylene blue) injection USP, for intravenous use
Initial U.S. Approval: 2016
WARNING: SEROTONIN SYNDROME WITH CONCOMITANT USE OF SEROTONERGIC DRUGS
See full prescribing information for complete boxed warning
PROVAYBLUE ® may cause serious or fatal serotonergic syndrome when used in combination with serotonergic drugs. Avoid concomitant use ( 5.1, 7.1)
Indications and Usage for ProvayBlue
PROVAYBLUE ® (methylene blue) is an oxidation-reduction agent indicated for the treatment of pediatric and adult patients with acquired methemoglobinemia. This indication is approved under accelerated approval. Continued approval for this indication may be contingent upon verification of clinical benefit in subsequent trials. ( 1, 14)
ProvayBlue Dosage and Administration
- Administer 1 mg/kg intravenously over 5-30 minutes. ( 2.1)
- If methemoglobin level remains above 30% or if clinical symptoms persist, give a repeat dose of up to 1 mg/kg one hour after the first dose. ( 2.1)
- Administer a single dose of 1 mg/kg in patients with moderate or severe renal impairment. ( 2.2)
Dosage Forms and Strengths
50 mg/10 mL (5 mg/mL) single-dose ampule. ( 3)
10 mg/2 mL (5 mg/mL) single-dose ampule. ( 3)
50 mg/10 mL (5 mg/mL) single-dose vial. ( 3)
10 mg/2 mL (5 mg/mL) single-dose vial. ( 3)
Contraindications
PROVAYBLUE ® is contraindicated in the following conditions ( 4):
- Severe hypersensitivity to methylene blue
- Patients with glucose-6-phosphate dehydrogenase deficiency (G6PD) due to the risk of hemolytic anemia
Warnings and Precautions
- Hypersensitivity: If severe or life threatening allergic reaction occurs, discontinue PROVAYBLUE ®, treat the allergic reaction, and monitor until signs and symptoms resolve ( 5.2)
- Lack of Effectiveness: Consider alternative treatments if there is no resolution of methemoglobinemia after 2 doses ( 2.1, 5.3)
- Hemolytic Anemia: Discontinue PROVAYBLUE ® and transfuse ( 5.4)
- Interference with In-Vivo Monitoring Devices: Use methods other than pulse oximetry to assess oxygen saturation ( 5.5)
- Effects on Ability to Drive and Operate Machinery: Advise patients to refrain from these activities until neurologic and visual symptoms have resolved ( 5.6)
Adverse Reactions/Side Effects
The most commonly reported adverse reactions (≥10%) are pain in extremity, chromaturia, dysgeusia, feeling hot, dizziness, hyperhidrosis, nausea, skin discoloration and headache. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact American Regent at 1-800-734-9236 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
- Pregnancy: Only use during pregnancy if the potential benefit justifies the potential risk to the fetus. ( 8.1)
- Lactation: Discontinue breast-feeding for up to 8 days after treatment. ( 8.2).
- Hepatic Impairment: Monitor patients longer for toxicity and drug interactions due to delayed clearance. ( 8.7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2021
Related/similar drugs
methylene blueFull Prescribing Information
WARNING: SEROTONIN SYNDROME WITH CONCOMITANT USE OF SEROTONERGIC DRUGS
PROVAYBLUE ® may cause serious or fatal serotonergic syndrome when used in combination with serotonergic drugs. Avoid concomitant use of PROVAYBLUE ® with selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), and monoamine oxidase inhibitors ( 5.1, 7.1). [see Warnings and Precautions ( 5.1) and Drug Interactions ( 7.1)]
1. Indications and Usage for ProvayBlue
PROVAYBLUE ® USP is indicated for the treatment of pediatric and adult patients with acquired methemoglobinemia.
This indication is approved under accelerated approval. Continued approval for this indication may be contingent upon verification of clinical benefit in subsequent trials [see Clinical Studies (14.1)].
2. ProvayBlue Dosage and Administration
2.1 Dosage and Administration
- Ensure patent venous access prior to administration of PROVAYBLUE®. Do not administer PROVAYBLUE® subcutaneously.
- Monitor vital signs, electrocardiogram and methemoglobin levels during treatment with PROVAYBLUE® and through resolution of methemoglobinemia.
- Administer PROVAYBLUE® 1 mg/kg intravenously over 5-30 minutes.
- If the methemoglobin level remains greater than 30% or if clinical signs and symptoms persist, a repeat dose of PROVAYBLUE® 1 mg/kg may be given one hour after the first dose.
- If methemoglobinemia does not resolve after 2 doses of PROVAYBLUE®, consider initiating alternative interventions for treatment of methemoglobinemia.
2.2 Recommended Dosage for Renal Impairment
- The recommended dosage of PROVAYBLUE® in patients with moderate or severe renal impairment (eGFR 15-59 mL/min/1.73 m2) is a single dose of 1 mg/kg.
- If the methemoglobin level remains greater than 30% or if the clinical symptoms persist 1 hour after dosing, consider initiating alternative interventions for the treatment of methemoglobinemia.
2.3 Preparation and Storage
Each mL of PROVAYBLUE ® contains 5 mg methylene blue.
Each 10 mL ampule and vial of PROVAYBLUE ® contains 50 mg methylene blue. Each 2 mL ampule and vial of PROVAYBLUE® contains 10 mg methylene blue.
PROVAYBLUE ® is hypotonic and may be diluted before use in a solution of 50 mL 5% Dextrose Injection in order to avoid local pain, particularly in the pediatric population. Use the diluted solution immediately after preparation.
Avoid diluting with sodium chloride solutions, because it has been demonstrated that chloride reduces the solubility of methylene blue.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Keep the ampule and the vial in the original package to protect from light.
Discard unused portion.
3. Dosage Forms and Strengths
Injection: 50 mg/10 mL (5 mg/mL) or 10 mg/2 mL (5 mg/mL) clear dark blue solution in single-dose ampules or single-dose vials.
4. Contraindications
PROVAYBLUE ® is contraindicated in the following conditions:
- Severe hypersensitivity reactions to methylene blue or any other thiazine dye [see Warnings and Precautions ( 5.2)] .
- Patients with glucose-6-phosphate dehydrogenase deficiency (G6PD) due to the risk of hemolytic anemia [see Warnings and Precautions ( 5.3, 5.4)]
5. Warnings and Precautions
5.1 Serotonin Syndrome with Concomitant Use of Serotonergic Drugs
The development of serotonin syndrome has been reported with use of methylene blue class products. Most reports have been associated with concomitant use of serotonergic drugs (e.g., selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors). Some of the reported cases were fatal. Symptoms associated with serotonin syndrome may include the following combination of signs and symptoms: mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, and hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, and incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Avoid concomitant use of PROVAYBLUE ® with serotonergic drugs.
Patients treated with PROVAYBLUE ® should be monitored for the emergence of serotonin syndrome. If symptoms of serotonin syndrome occur, discontinue use of PROVAYBLUE ®, and initiate supportive treatment. Inform patients of the increased risk of serotonin syndrome and advise them to not to take serotonergic drugs within 72 hours after the last dose of PROVAYBLUE ®[see Drug Interactions ( 7), Patient Counseling Information ( 17)] .
5.2 Hypersensitivity
Anaphylactic reactions to methylene blue class products have been reported. Patients treated with PROVAYBLUE ® should be monitored for anaphylaxis. If anaphylaxis or other severe hypersensitivity reactions (e.g., angioedema, urticaria, bronchospasm) should occur, discontinue use of PROVAYBLUE ® and initiate supportive treatment. PROVAYBLUE ® is contraindicated in patients who have experienced anaphylaxis or other severe hypersensitivity reactions to a methylene blue class product in the past.
5.3 Lack of Effectiveness
Methemoglobinemia may not resolve or may rebound after response to treatment with PROVAYBLUE ® in patients with methemoglobinemia due to aryl amines such as aniline or sulfa drugs such as dapsone. Monitor response to therapy with PROVAYBLUE ® through resolution of methemoglobinemia. If methemoglobinemia does not respond to 2 doses of PROVAYBLUE ® or if methemoglobinemia rebounds after a response, consider additional treatment options [see Dosage and Administration ( 2.2)] .
Patients with glucose-6-phosphate dehydrogenase deficiency may not reduce PROVAYBLUE ® to its active form in vivo. PROVAYBLUE ® may not be effective in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.
5.4 Hemolytic Anemia
Hemolysis can occur during treatment of methemoglobinemia with PROVAYBLUE ®. Laboratory testing may show Heinz bodies, elevated indirect bilirubin and low haptoglobin, but the Coombs test is negative. The onset of anemia may be delayed 1 or more days after treatment with PROVAYBLUE ®. The anemia may require red blood cell transfusions [see Adverse Reactions ( 6.1)]. Use the lowest effective number of doses of PROVAYBLUE ® to treat methemoglobinemia. Discontinue PROVAYBLUE ® and consider alternative treatments of methemoglobinemia if severe hemolysis occurs.
Treatment of patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency with PROVAYBLUE ® may result in severe hemolysis and severe anemia. PROVAYBLUE ® is contraindicated for use in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency [see Contraindications ( 4)].
5.5 Interference with In Vivo Monitoring Devices
- Inaccurate Pulse Oximeter Readings
The presence of methylene blue in the blood may result in an underestimation of the oxygen saturation reading by pulse oximetry. If a measure of oxygen saturation is required during or shortly after infusion of PROVAYBLUE ®, it is advisable to obtain an arterial blood sample for testing by an alternative method.
- Bispectral index monitor
A fall in the Bispectral Index (BIS) has been reported following administration of methylene blue class products. If PROVAYBLUE ® is administered during surgery, alternative methods for assessing the depth of anesthesia should be employed.
5.6 Effects on Ability to Drive and Operate Machinery
Treatment with PROVAYBLUE ® may cause confusion, dizziness and disturbances in vision [see Adverse Reactions ( 6)] . Advise patients to refrain from driving or engaging in hazardous occupations or activities such as operating heavy or potentially dangerous machinery until such adverse reactions to PROVAYBLUE ® have resolved.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Serotonin Syndrome with Concomitant Use of Serotonergic Drugs [see Warnings and Precautions ( 5.1)]
- Anaphylaxis [see Warnings and Precautions ( 5.2)]
- Lack of Effectiveness [see Warnings and Precautions ( 5.3)]
- Hemolytic Anemia [see Warnings and Precautions ( 5.4)]
- Interference with In-Vivo Monitoring Devices [see Warnings and Precautions ( 5.5)]
- Effects on Ability to Drive and Operate Machinery [see Warnings and Precautions ( 5.6)]
- Interference with Laboratory Tests [see Warnings and Precautions ( 5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of PROVAYBLUE ® was determined in 82 healthy adults of median age of 36 years (range, 19-55 years); 54% were male, and 68% were white. Each individual in the safety population received a single dose of PROVAYBLUE ® 2 mg/kg intravenously. There was one serious adverse reaction reported (syncope due to sinus pauses of 3-14 seconds). The most common (≥2%) moderate or severe adverse reactions were pain in the extremity (56%), headache (7%), feeling hot (6%), syncope (4%), back pain (2%), hyperhidrosis (2%) and nausea (2%). Table 1 lists the adverse reactions of any severity that occurred in at least 2% of individuals who received PROVAYBLUE ®.
| Adverse Reaction | Any Grade TEAE
(n=82) | Moderate-
Severe TEAE (n=82) |
||
| Pain in extremity | 69 | 84% | 46 | 56% |
| Chromaturia | 61 | 74% | 0 | |
| Dysgeusia | 16 | 20% | 1 | 1% |
| Feeling hot | 14 | 17% | 5 | 6% |
| Dizziness | 13 | 16% | 4 | 5% |
| Hyperhidrosis | 11 | 13% | 2 | 2% |
| Nausea | 11 | 13% | 2 | 2% |
| Skin discoloration | 11 | 13% | 0 | |
| Headache | 8 | 10% | 6 | 7% |
| Musculoskeletal pain | 7 | 9% | 0 | |
| Paresthesia oral | 7 | 9% | 0 | |
| Paresthesia | 7 | 9% | 0 | |
| Infusion site pain | 5 | 6% | 1 | 1% |
| Feeling cold | 5 | 6% | 0 | |
| Pallor | 4 | 5% | 0 | |
| Dermatitis contact | 4 | 5% | 0 | |
| Syncope | 3 | 4% | 3 | 4% |
| Influenza like illness | 3 | 4% | 1 | 1% |
| Pruritus | 3 | 4% | 1 | 1% |
| Anxiety | 3 | 4% | 0 | |
| Decreased appetite | 3 | 4% | 0 | |
| Chest discomfort | 3 | 4% | 0 | |
| Back pain | 2 | 2% | 2 | 2% |
| Cold sweat | 2 | 2% | 1 | 1% |
| Dizziness postural | 2 | 2% | 1 | 1% |
| Muscle spasms | 2 | 2% | 1 | 1% |
| Presyncope | 2 | 2% | 1 | 1% |
| Vomiting | 2 | 2% | 1 | 1% |
| Arthralgia | 2 | 2% | 1 | 1% |
| Chills | 2 | 2% | 0 | |
| Diarrhea | 2 | 2% | 0 | |
| Discomfort | 2 | 2% | 0 | |
| Dyspnea | 2 | 2% | 0 | |
| Erythema | 2 | 2% | 0 | |
| Hypoesthesia oral | 2 | 2% | 0 | |
| Infusion site discomfort | 2 | 2% | 0 | |
| Limb discomfort | 2 | 2% | 0 | |
| Oral discomfort | 2 | 2% | 0 | |
| Catheter site pain | 2 | 2% | 0 | |
| Ecchymosis | 2 | 2% | 0 | |
Other adverse reactions reported to occur following administration of methylene blue class products include the following:
Blood and lymphatic system disorders: hemolytic anemia, hemolysis, hyperbilirubinemia, methemoglobinemia
Cardiac disorders: palpitations, tachycardia
Eye disorders: eye pruritus, ocular hyperemia, vision blurred
Gastrointestinal disorders: abdominal pain lower, dry mouth, flatulence, glossodynia, tongue eruption
General disorders and administration site conditions: death, infusion site extravasation, infusion site induration, infusion site pruritus, infusion site swelling, infusion site urticaria, peripheral swelling, thirst
Investigations: elevated liver enzymes
Musculoskeletal and connective tissue disorders: myalgia
Renal and urinary disorders: dysuria
Respiratory, thoracic and mediastinal disorders: nasal congestion, oropharyngeal pain, rhinorrhea, sneezing
Skin and subcutaneous tissue disorders: necrotic ulcer, papule, phototoxicity
Vascular disorders: hypertension
7. Drug Interactions
7.1 Serotonergic Drugs
Avoid concomitant use of PROVAYBLUE® with medicinal products that enhance serotonergic transmission including SSRIs (selective serotonin reuptake inhibitors), MAO inhibitors, bupropion, buspirone, clomipramine, mirtazapine and venlafaxine; because of the potential for serious CNS reactions, including potentially fatal serotonin syndrome. Although the mechanism is not clearly understood, literature reports suggest inhibition of MAO by methylene blue may be involved. If the intravenous use of PROVAYBLUE® cannot be avoided in patients treated with serotonergic medicinal products, choose the lowest possible dose and observe closely the patient for CNS effects for up to 4 hours after administration [see Warnings and Precautions ( 5.1) and Clinical Pharmacology ( 12.3)].
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
PROVAYBLUE ® may cause fetal harm when administered to a pregnant woman. Intra-amniotic injection of pregnant women with a methylene blue class product during the second trimester was associated with neonatal intestinal atresia and fetal death. Methylene blue produced adverse developmental outcomes in rats and rabbits when administered orally during organogenesis at doses at least 32 and 16 times, respectively, the clinical dose of 1 mg/kg [see Data] . Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2-4% and 15-20%, respectively.
Clinical Considerations
Fetal/neonatal adverse reactions
Intra-amniotic injection of a methylene blue class product hours to days prior to birth can result hyperbilirubinemia, hemolytic anemia, skin staining, methemoglobinemia, respiratory distress and photosensitivity in the newborn. Following administration of PROVAYBLUE ® to a pregnant woman at term, observe the newborn for these adverse reactions and institute supportive care.
Data
Animal Data
Methylene blue was administered orally to pregnant rats at doses of 50 to 350 mg/kg/day, during the period of organogenesis. Maternal and embryofetal toxicities were observed at all doses of methylene blue and were most evident at the 200 and 350 mg/kg/day doses. Maternal toxicity consisted of increased spleen weight. Embryo-fetal toxicities included reduced fetal weight, post-implantation loss, edema, and malformations including enlarged lateral ventricles. The dose of 200 mg/kg (1200 mg/m 2) in rats is approximately 32 times a clinical dose of 1 mg/kg based on body surface area.
Methylene blue was administered orally to pregnant rabbits at doses of 50, 100, or 150 mg/kg/day, during the period of organogenesis. Maternal death was observed at the methylene blue dose of 100 mg/kg. Embryofetal toxicities included spontaneous abortion at all dose levels and a malformation (umbilical hernia) at the 100 and 150 mg/kg/day doses. The dose of 50 mg/kg (600 mg/m 2) in rabbits is approximately 16 times a clinical dose of 1 mg/kg based on body surface area.
8.2 Lactation
Risk Summary
There is no information regarding the presence of methylene blue in human milk, the effects on the breastfed infant, or the effects on milk production. Because of the potential for serious adverse reactions including genotoxicity, discontinue breast-feeding during and for up to 8 days after treatment with PROVAYBLUE ®[see Clinical Pharmacology ( 12.3)] .
8.4 Pediatric Use
The safety and effectiveness of PROVAYBLUE ® have been established in pediatric patients. Use of PROVAYBLUE ® is supported by two retrospective case series that included 2 pediatric patients treated with PROVAYBLUE ® and 12 treated with another methylene blue class product. The case series included pediatric patients in the following age groups: 3 neonates (less than 1 month), 4 infants (1 month up to less than 2 years), 4 children (2 years up to less than 12 years), and 3 adolescents (12 years to less than 17 years). The efficacy outcomes were consistent across pediatric and adult patients in both case series [see Clinical Studies ( 14)].
8.5 Geriatric Use
The retrospective case series included 3 patients age 65 years and over treated with PROVAYBLUE ® (or a bioequivalent formulation) and 5 treated with another methylene blue class product. The efficacy outcomes were consistent across adult and elderly patients in both case series [see Clinical Studies ( 14)] . This drug is known to be substantially excreted by the kidney, so the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, treatment of methemoglobinemia in these patients should use the lowest number of doses needed to achieve a response [see Dosage and Administration ( 2)] .
8.6 Renal Impairment
Methylene blue concentrations increased in subjects with renal impairment (eGFR 15 to 89 mL/min/1.73m2) significantly [see Clinical Pharmacology ( 12.3)]. Adjust PROVAYBLUE® dosage in patients with moderate or severe renal impairment (eGFR 15 to 59 mL/min/1.73 m2) [see Dosage and Administration ( 2.2)]. No dose adjustment is recommended in patients with mild renal impairment (eGFR 60 – 89 mL/min/1.73 m2).
10. Overdosage
Hypotension, wheezing and reduced oxygenation have been reported in patients who received methylene blue class products in single doses of 3 mg/kg or more.
Administration of large intravenous doses (cumulative dose ≥ 7 mg/kg ) of a methylene blue class product caused nausea, vomiting, precordial pain, dyspnea, tachypnea, chest tightness, tachycardia, apprehension, tremor, mydriasis, blue staining of the urine, the skin and mucous membranes, abdominal pain, dizziness, paresthesia, headache, confusion, mild methemoglobinemia (up to 7%) and electrocardiogram changes (T-wave flattening or inversion). These effects lasted 2-12 hours following administration.
A severe overdosage (single dose of 20 mg/kg or more) of a methylene blue class product caused severe intravascular hemolysis, hyperbilirubinemia and death.
In case of overdose of PROVAYBLUE ®, maintain the patient under observation until signs and symptoms have resolved, monitor for cardiopulmonary, hematologic and neurologic toxicities, and institute supportive measures as necessary.
11. ProvayBlue Description
Methylene blue is an oxidation-reduction agent.
Its chemical name is 3,7-bis(dimethylamino)phenothiazin-5-ium, chloride hydrate. The molecular formula of methylene blue is C16H18ClN3S.xH2O and its molecular weight of 319.86 g/mol for the anhydrous form. The structural formula of methylene blue is:
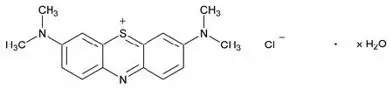
PROVAYBLUE® (methylene blue) injection is a sterile solution intended for intravenous administration. Each mL of solution contains 5 mg methylene blue and water for injection. PROVAYBLUE® is a clear dark blue solution with a pH value between 3.0 and 4.5. The osmolality is between 10 and 15 mOsm/kg. PROVAYBLUE® (methylene blue) injection strength is expressed in terms of trihydrate.
12. ProvayBlue - Clinical Pharmacology
12.1 Mechanism of Action
Methylene blue is a water soluble thiazine dye that promotes a non-enzymatic redox conversion of metHb to hemoglobin. In situ, methylene blue is first converted to leucomethylene blue (LMB) via NADPH reductase. It is the LMB molecule which then reduces the ferric iron of metHb to the ferrous state of normal hemoglobin.
12.2 Pharmacodynamics
Low concentrations of methylene blue speeds up the in vivo conversion of methemoglobin to hemoglobin. Methylene blue has been observed to stain tissues selectively. The exposure-response or –safety relationship for methylene is unknown.
12.3 Pharmacokinetics
The mean (CV%) Cmax and AUC of methylene blue 2,917 ng/mL (39%) and 13977 ng.hr/mL (21%) following a 2 mg/kg dose administered as a 5-minute intravenous infusion.
Distribution
The mean± standard deviation steady state volume of distribution of a 2 mg/kg dose of PROVAYBLUE ® was 255 L ± 58. The mean plasma protein binding of methylene blue is approximately 94% in vitro. Methylene blue exhibits concentration-dependent partitioning into blood cells in vitro. The blood-to-plasma ratio was 5.1±2.8 at 5 minutes from the start of a 2 mg/kg dose administered as a 5-minute intravenous infusion and reached a plateau of 0.6 at 4 hours in a clinical study. Methylene Blue is a substrate for the P-glycoprotein (P-gp, ABCB1) transporter, but not for BCRP or OCT2 in vitro.
Metabolism
Methylene blue is metabolized by CYPs 1A2, 2C19 and 2D6 in vitro; however, the predominant in vitro pathway appears to be UGT-mediated conjugation by multiple UGT enzymes, including UGT1A4 and UGT1A9.
Azure B, which is a minor impurity in methylene blue, is also formed in humans as a metabolite of methylene blue, with an overall drug/metabolite AUC ratio of greater than 6:1. Azure B has 8-fold lower potency than methylene blue.
Specific Populations
Renal Impairment
After a single 1 mg/kg dose of PROVAYBLUE®, AUC0-96h increased by 52%, 116%, and 192% in subjects with mild (estimated glomerular filtration rate (eGFR) 60 – 89 mL/min/1.73 m2), moderate (eGFR 30-59 mL/min/1.73m2), and severe (eGFR 15-29 mL/min/1.732m2) renal impairment, respectively. Cmax increased by 42%, 34%, and 15% in subjects with mild, moderate, and severe renal impairment respectively [see Dosage and Administration ( 2.2) and Use in Specific Populations ( 8.6)]. The half-life was unchanged in patients with mild to moderate renal impairment.
The AUC0-96h of Azure B after a single 1 mg/kg dose increased by 29%, 94%, and 339% in subjects with mild (estimated glomerular filtration rate (eGFR) 60 – 89 mL/min/1.73 m2), moderate (eGFR 30-59 mL/min/1.73m2), and severe (eGFR 15-29 mL/min/1.732m2) renal impairment, respectively. Cmax increased by 23%, 13%, and 65% in subjects with mild, moderate, and severe renal impairment, respectively [see Dosage and Administration ( 2.2) and Use in Specific Populations ( 8.6)]
Drug Interactions Studies
Clinical Studies:
The coadministration of 2 mg/kg dose of PROVAYBLUE® with midazolam (a CYP3A4 substrate), caffeine (a CYP1A2 substrate), warfarin (a CYP2C9 substrate), and dextromethorphan (a CYP2D6 substrate) in a cocktail study did not affect the exposure of these substrates compared to their exposure without PROVAYBLUE® administration.
In Vitro Studies:
Cytochrome P450 (CYP450) Enzymes:
Methylene blue inhibits CYP isozymes 1A2, 2B6, 2C8, 2C9, 2C19, 2D6 and 3A4/5. Possible time-dependent inhibition of CYP2C9, CYP2D6 and CYP3A4/5 (testosterone as substrate) was also observed. Methylene blue induces CYP1A2 but does not induce CYP2B6 or CYP3A4.
UDP-Glucuronosyltransferase (UGT):
Methylene blue inhibits UGT1A9 and UGT1A4, but did not significantly inhibit UGTs 1A1, 1A3, 1A6, 2B7 or 2B15.
Transporter:
Methylene blue is both a substrate for and an inhibitor of P-gp but is not a substrate for BCRP or OCT2 in vitro. Methylene blue is not a significant inhibitor of BCRP, OAT1, OAT3, OAT1B1 or OAT1B3. Methylene blue inhibits OCT2, MATE1 and MATE2-K.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a two-year carcinogenicity study, rats were administered oral doses of methylene blue at 5, 25, or 50 mg/kg. Methylene blue caused pancreatic islet adenomas or carcinomas (combined) in male rats. In a two-year carcinogenicity study, mice were administered oral doses of methylene blue at 2.5, 12.5, or 25 mg/kg. There were no drug-related neoplastic findings in mice.
Methylene blue was genotoxic in gene mutation assays in bacteria (Ames test), and in an in vitro sister chromatid exchange test and an in vitro chromosomal aberration test in Chinese hamster ovary (CHO) cells. Methylene blue was negative for micronucleus induction in bone marrow or peripheral blood collected from mice treated with methylene blue.
Fertility studies with methylene blue have not been conducted. In vitro, methylene blue reduced motility of human sperm in a concentration dependent manner.
14. Clinical Studies
14.1 Treatment of Acquired Methemoglobinemia
The efficacy of PROVAYBLUE ® was assessed on the basis of a methemoglobin decrease of at least 50% within 1 hour after intravenous administration of 1 – 2 mg/kg PROVAYBLUE ® (or a bioequivalent formulation) in 6 patients identified by retrospective chart review or literature search. The 6 patients included 3 males and 3 females of median age 54 years (range, 6 days to 69 years). The median methemoglobin level at baseline was 37% (range, 11% to 47%). All 6 (100%) patients had a decrease in methemoglobin by at least 50% within 1 hour after treatment.
An additional 41 cases of treatment of methemoglobinemia with a methylene blue class product were identified in the published literature. These cases included 24 males and 17 females of median age 33 years (range, 9 days to 80 years). The median methemoglobin level at baseline was 40% (range, 10% to 98%). Of these 41 patients, 37 (90%) had a methemoglobin decrease of at least 50% within 1 hour after intravenous administration of the methylene blue class product.
In a combined analysis of all 47 patients treated intravenously with PROVAYBLUE ® (or a bioequivalent formulation) or with another methylene blue class product, there was no difference in response rate by dose. The methemoglobin decreased by at least 50% within 1 hour of infusion for 15/17 (88%) of patients treated with 1 mg/kg, 12/13 (92%) treated with 2 mg/kg and 16/17 (94%) treated with a different dose or for those whose dose was not reported.
16. How is ProvayBlue supplied
PROVAYBLUE ® is supplied in 10 mL and 2 mL single-dose ampules or single-dose vials. Each 10 mL ampule and vial contains 50 mg of methylene blue as a clear dark blue solution. Each 2 mL ampule and vial contains 10 mg of methylene blue as a clear dark blue solution. A box contains five ampules or vials.
Box of 5 ampules of 50 mg/10 mL: NDC 0517-0374-05
Box of 5 ampules of 10 mg/2 mL: NDC 0517-0125-05
Box of 5 vials of 50 mg/10 mL: NDC 0517-0381-05
Box of 5 vials of 10 mg/2 mL: NDC 0517-0371-05
Storage:
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature]
Any unused product or waste material should be disposed of in accordance with local practice.
Do not refrigerate or freeze.
Keep the ampule or the vial in the original package to protect from light.
17. Patient Counseling Information
Serotonin Syndrome
Advise patients of the possibility of serotonin syndrome, especially with concomitant use of serotonergic agents such as medications to treat depression and migraines. Advise patients to seek immediate medical attention if the following symptoms occur after treatment with PROVAYBLUE ®: changes in mental status, autonomic instability, or neuromuscular symptoms with or without gastrointestinal symptoms [see Warnings and Precautions ( 5.1)].
Pregnancy
Advise pregnant women of the potential risk to the fetus with the use of PROVAYBLUE ® during pregnancy [see Use in Specific populations ( 8.1)].
Driving and Using Machines
Advise patients to avoid driving and use of machines during treatment with PROVAYBLUE ®. Driving can be affected as a result of a confusional state, dizziness and possible eye disturbances [see Warnings and Precautions ( 5.6)] .
Phototoxicity
Advise patients to take protective measures against exposure to light, because phototoxicity may occur after administration of methylene blue [see Adverse Reactions ( 6.1)] .
Skin and Body Fluid Blue Discoloration
Advise patients that PROVAYBLUE ® may cause a blue discoloration of the skin and body fluids [see Adverse Reactions ( 6.1)].
Manufactured for:
PROVEPHARM SAS
22 rue Marc Donadille
13013 Marseille, France
Ampules manufactured by:
CENEXI
52 rue Marcel et Jacques Gaucher
94120 Fontenay sous Bois, FRANCE
Vials manufactured by: CENEXI HSC
2 rue Louis Pasteur
14200 Hérouville-Saint-Clair, France
Distributed by:
American Regent, Inc.
Shirley, NY 11967
Questions? : 1-800-734-9236
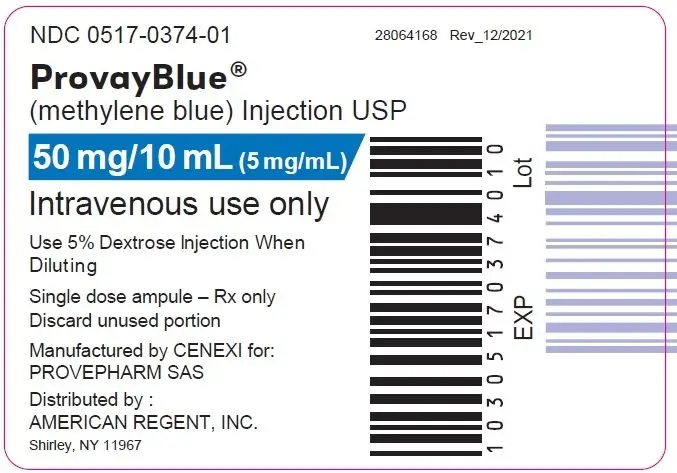
Principal Display Panel - 50 mg/10 mL (5 mg/mL) Ampule Label
NDC 0517-0374-01
ProvayBlue®
(methylene blue) Injection USP
50 mg/10 mL (5 mg/mL)
Intravenous use only
Use 5% Dextrose Injection When
Diluting
Single dose ampule - Rx only
Discard unused portion
Manufactured by CENEXI for:
PROVEPHARM SAS
Distributed by:
AMERICAN REGENT, INC
Shirley, NY 11967
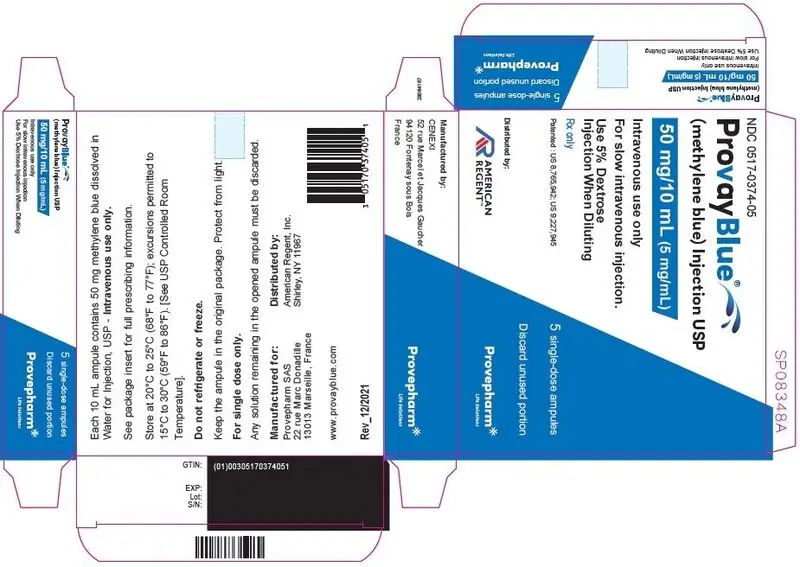
Principal Display Panel - 50 mg/10 mL (5 mg/mL) Carton Label
NDC 0517-0374-05
ProvayBlue®
(methylene blue) Injection USP
50 mg/10 mL (5 mg/mL)
Intravenous use only
For slow intravenous injection.
Use 5% Dextrose
Injection When Diluting
Rx only
Patented : US 8,765,942; US 9,227,945
5 single-dose ampules
Discard unused portion
Distributed by:
AMERICAN
REGENT
Provepharm
Life Solutions
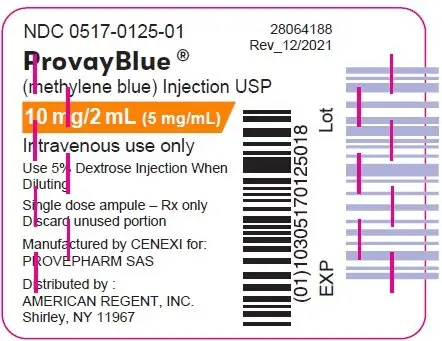
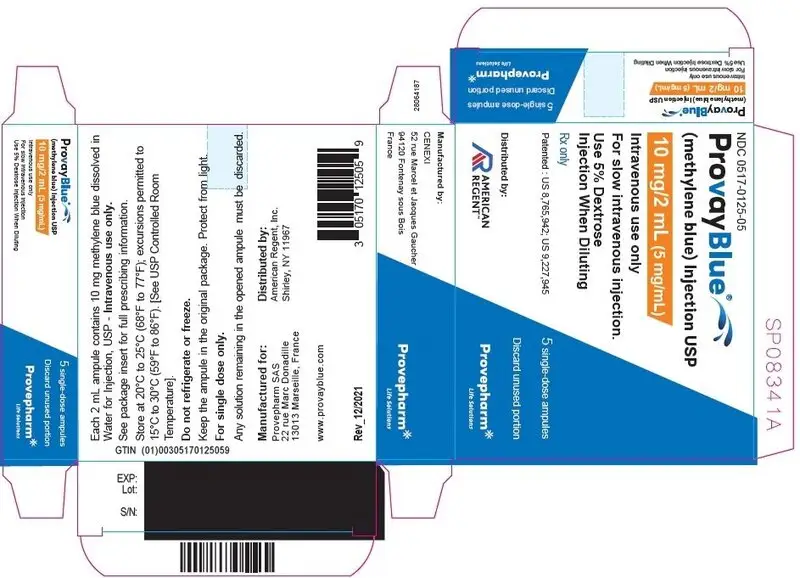
Principal Display Panel - 10 mg/2 mL (5 mg/mL) Ampule Label
NDC 0517-0125-01
ProvayBlue®
(methylene blue) Injection USP
10 mg/2 mL (5 mg/mL)
Intravenous use only
Use 5% Dextrose Injection When
Diluting
Single dose ampule - Rx only
Discard unused portion
Manufactured by CENEXI for:
PROVEPHARM SAS
Distributed by:
AMERICAN REGENT, INC.
Shirley, NY 11967
Principal Display Panel - 10 mg/2 mL (5 mg/mL) Carton Label
NDC 0517-0125-05 5 ampules
ProvayBlue®
(methylene blue) Injection USP
10 mg/2 mL (5 mg/mL)
Intravenous use only
For slow intravenous injection.
Use 5% Dextrose
Injection When Diluting
Rx only
Patented: US 8,765,942; US 9,227,945
5 single-dose ampules
Discard unused portion
Distributed by:
AMERICAN
REGENT
Provepharm
Life Solutions
Principal Display Panel - 50 mg/10 mL (5 mg/mL) Single-Dose Vial Label
NDC 0517-0381-01
ProvayBlue®
(methylene blue) Injection USP
50 mg/10 mL (5 mg/mL)
Intravenous use only
Use 5% Dextrose in Water When Diluting
Single dose vial - Rx only
Discard unused portion
Manufactured by CENEXI for:
PROVEPHARM SAS
Distributed by:
AMERICAN REGENT, INC.
Shirley, NY 11967

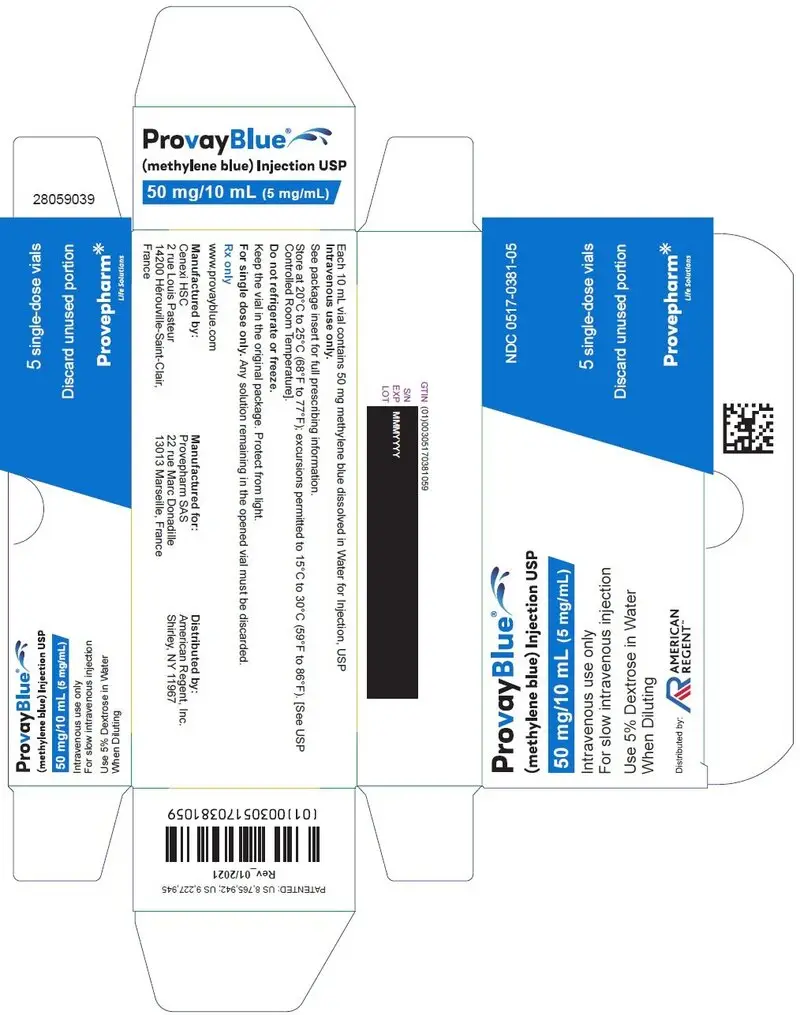
Principal Display Panel - 50 mg/10 mL (5 mg/mL) Single-Dose Vial Carton Label
NDC 0517-0381-05
ProvayBlue®
(methylene blue) Injection USP
50 mg/10 mL (5 mg/mL)
Intravenous use only
For slow intravenous injection
Use 5% Dextrose in Water When Diluting
Distributed by:
AMERICAN REGENT
5 single-dose vials
Discard unused portion
Provepharm Life Solutions
Principal Display Panel - 10 mg/2 mL (5 mg/mL) Single-Dose Vial Label
NDC 0517-0371-01
ProvayBlue®
(methylene blue) Injection USP
10 mg/2 mL (5 mg/mL)
Intravenous use only
Use 5% Dextrose in Water When Diluting
Single dose vial - Rx only
Discard unused portion
Manufactured by CENEXI for:
PROVEPHARM SAS
Distributed by:
AMERICAN REGENT, INC.
Shirley, NY 11967

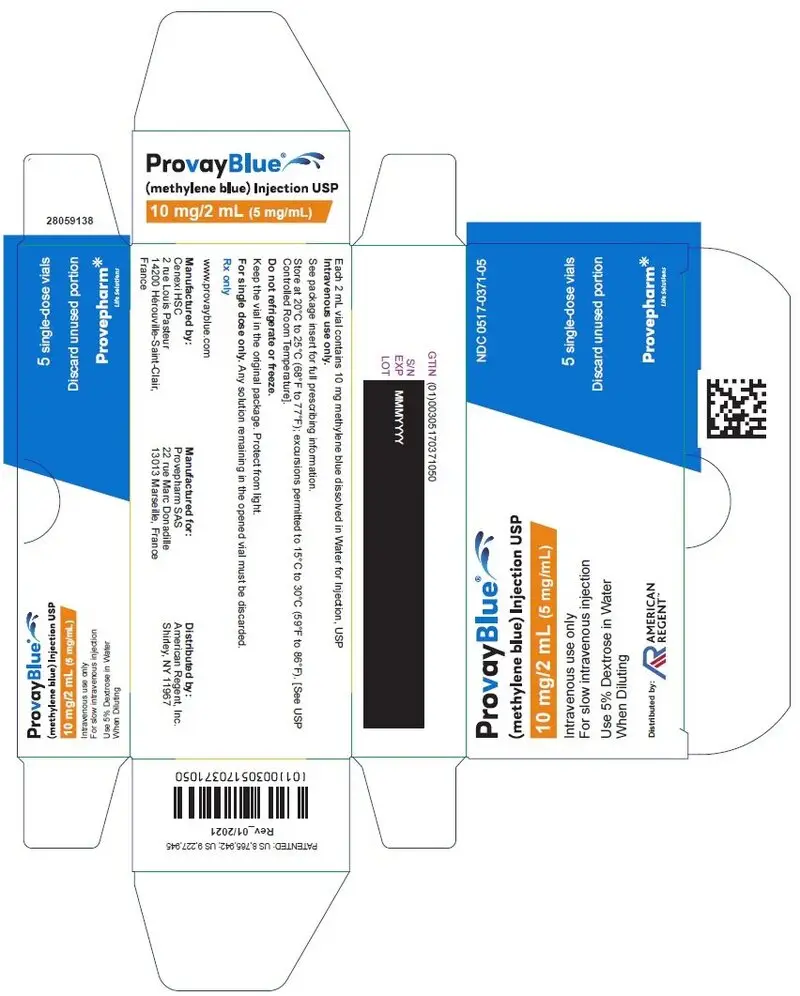
Principal Display Panel - 10 mg/2 mL (5 mg/mL) Single-Dose Vial Carton Label
NDC 0517-0371-05
ProvayBlue®
(methylene blue) Injection USP
10 mg/2 mL (5 mg/mL)
Intravenous use only
For slow intravenous injection
Use 5% Dextrose in Water When Diluting
Distributed by:
AMERICAN REGENT
5 single-dose vials
Discard unused portion
Provepharm Life Solutions
| PROVAYBLUE
methylene blue injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PROVAYBLUE
methylene blue injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PROVAYBLUE
methylene blue injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PROVAYBLUE
methylene blue injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - American Regent, Inc. (002033710) |
| Registrant - Provepharm SAS (296789410) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cenexi | 573309239 | manufacture(0517-0374, 0517-0125) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CENEXI HSC | 268155718 | manufacture(0517-0381, 0517-0371) | |




