Drug Detail:Sinuva (Mometasone nasal [ moe-met-a-sone ])
Drug Class: Nasal steroids
Highlights of Prescribing Information
SINUVA (mometasone furoate) sinus implant
Initial U.S. Approval: 1987
Indications and Usage for Sinuva
SINUVA Sinus Implant is a corticosteroid-eluting implant indicated for the treatment of chronic rhinosinusitis with nasal polyps in adult patients ≥ 18years of age who have had ethmoid sinus surgery. (1)
Sinuva Dosage and Administration
- The SINUVA Sinus Implant is loaded into a Delivery System and placed in the ethmoid sinus under endoscopic visualization. The Implant may be left in the sinus to gradually release the corticosteroid over 90 days. Remove the implant by 90 days or earlier at the physician's discretion using standard surgical instruments. (2.2)
- To be inserted by physicians trained in otolaryngology. (2.3)
Dosage Forms and Strengths
Implant: 1350 mcg of mometasone furoate and a sterile Delivery System. (3)
Contraindications
Patients with known hypersensitivity to mometasone furoate and any of the ingredients of the SINUVA Sinus Implant. (4)
Warnings and Precautions
- Monitor nasal mucosa adjacent to the SINUVA Sinus Implant for any signs of bleeding (epistaxis), irritation, infection, or perforation. Avoid use in patients with nasal ulcers or trauma. (5.1)
- Monitor patients with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts closely. (5.2)
- Hypersensitivity reactions, including rash, pruritus, and angioedema, have been reported with use of corticosteroids. (5.3)
- Potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infection; or ocular herpes simplex. More serious or even fatal course of chickenpox or measles in susceptible patients. Use caution in patients with the above because of the potential for worsening of these infections. (5.4)
- If corticosteroid effects such as hypercorticism and adrenal suppression appear in patients, consider sinus implant removal. (5.5)
Adverse Reactions/Side Effects
The most common adverse reactions (in more than 1% of subjects) were bronchitis, nasopharyngitis, otitis media, headache, presyncope, asthma, and epistaxis. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Intersect ENT at 1-866 531-6004 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2023
Related/similar drugs
prednisone, fluticasone nasal, cetirizine, loratadine, promethazine, Zyrtec, mometasone nasalFull Prescribing Information
1. Indications and Usage for Sinuva
SINUVA Sinus Implant is indicated for the treatment of chronic rhinosinusitis with nasal polyps in adult patients ≥18 years of age who have had ethmoid sinus surgery.
2. Sinuva Dosage and Administration
2.1 Recommended Dosage
The recommended dosage is one SINUVA Sinus Implant (1350 mcg of mometasone furoate) placed in an ethmoid sinus [see Dosage and Administration (2.3)]. The SINUVA Sinus Implant may be left in the sinus to gradually release the corticosteroid over 90 days. Remove the SINUVA Sinus Implant by 90 days or earlier at the physician's discretion [see Dosage and Administration (2.4)].
2.2 Health Care Provider Training
The SINUVA Sinus Implant is to be used by physicians trained in otolaryngology. Specialized training is not required for these physicians.
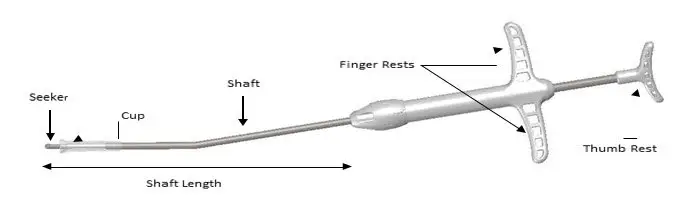
2.3 Placement of SINUVA Sinus Implant
The SINUVA Sinus Implant is designed for single patient use only. Do not reprocess or reuse.
- Do not use if the package is open, the package or product is damaged, or has evidence of gross contamination.
- Special care should be taken to avoid bending, twisting, or damaging the implant.
- The implant is not designed to be modified by the physician.
- The implant is not intended to be compressed and loaded into the Delivery System more than two times. The implant must be placed under endoscopic visualization.
Instructions to Secure the SINUVA Sinus Implant in the Crimper
If necessary, the Implant may be reloaded into the Crimper for a second time.
CAUTION: The SINUVA Sinus Implant should not be used if the second attempt to crimp is unsuccessful.
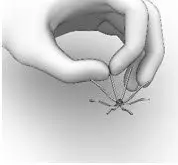
- Hold the SINUVA Sinus Implant by one end as shown in Figure 12.
Figure 12
- Holding the SINUVA Sinus Implant with the dome-shaped Cap positioned downward (Figure 13), place the Implant back into the Crimper.
Figure 13
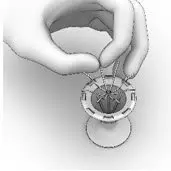
- Ensure that each Implant is secured in the Crimper by pressing down on the center of the Implant until all ends of the Implant are below the rim of the Crimper (Figure 14).
Figure 14
- Inspect the Implant and the Crimper to ensure that all the Implant ends are secured below the rim of the Crimper (Figure 15). Return to Implant Preparation Step 1 for instructions on how to load the re-secured implant into the delivery system.
Figure 15

3. Dosage Forms and Strengths
Implant: a sterile, single-use, bioabsorbable implant, coated with a formulation containing 1350 mcg mometasone furoate that is gradually released over 90 days.
4. Contraindications
Patients with known hypersensitivity to mometasone furoate, or to any of the copolymers of the SINUVA Sinus Implant [see Description (11)].
5. Warnings and Precautions
5.1 Local Nasal Adverse Reactions
Monitor nasal mucosa adjacent to the SINUVA Sinus Implant for any signs of bleeding (epistaxis), irritation, infection, or perforation. Avoid use in patients with nasal ulcers or trauma.
5.2 Glaucoma and Cataracts
Nasal steroids may result in development of glaucoma and/or cataracts. Glaucoma, cataracts, and clinically significant elevation of intraocular pressure were not observed in patients from the treatment group of one randomized controlled clinical study (N = 53) who underwent bilateral placement of SINUVA Sinus Implants. Close monitoring is warranted in patients with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts.
5.3 Hypersensitivity Reactions
Hypersensitivity reactions, including rash, pruritus, and angioedema have been reported with use of corticosteroids.
5.4 Immunosuppression and Risk of Infections
Persons who are using drugs that suppress the immune system, such as corticosteroids, including SINUVA Sinus Implant are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In such children or adults who have not had these diseases or who are not properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The safety and effectiveness of SINUVA Sinus Implant have not been established in pediatric patients less than 18 years of age and SINVA is not indicated for use in this population.
The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated (See the respective Prescribing Information for VZIG and IG). If chickenpox develops, treatment with antiviral agents may be considered.
Corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculosis infection of the respiratory tract; untreated systemic fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex.
5.5 Hypercorticism and Adrenal Suppression
Hypercorticism and adrenal suppression were not evaluated as part of the SINUVA Sinus Implant clinical program.
Since individual sensitivity to effects of cortisol production exists, physicians should consider this information when prescribing SINUVA Sinus Implant. Particular care should be taken in observing patients postoperatively or during periods of stress for evidence of inadequate adrenal response.
It is possible that systemic corticosteroid effects such as hypercorticism and adrenal suppression may appear in patients, particularly when systemic mometasone furoate is administered at higher than recommended doses over prolonged periods of time. If such effects occur, consider sinus implant removal.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Local Nasal Adverse Reactions [see Warnings and Precautions (5.1)]
- Glaucoma and Cataracts [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Immunosuppression and Risk of Infections [see Warnings and Precautions (5.4)]
- Hypercorticism and Adrenal Suppression [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of the SINUVA Sinus Implant was evaluated and demonstrated in 400 patients in 2 controlled, randomized, parallel group, single-blind studies. In Study 1, one-hundred (100) subjects were followed for 6 months. In Study 2, three-hundred (300) subjects were followed for 90 days. Of the 400 patients, 254 were assigned to the treatment group and underwent bilateral placement of SINUVA Sinus Implants in the ethmoid sinuses, totaling 2700 mcg of mometasone furoate, and 146 patients were assigned to the control group and underwent a sham procedure consisting of advancement of the Delivery System with the SINUVA Sinus Implant followed by removal without deployment. The Implants were removed by Day 60. All patients were required to use mometasone furoate nasal spray once daily (200 mcg of mometasone furoate) through Day 90.
Table 1 shows the common adverse reactions (in greater than 1% of subjects) that occurred more frequently in patients treated with SINUVA Sinus Implant compared to the control group.
| Study 1 & Study 2 Combined Data | ||
|---|---|---|
| Adverse Reaction | Treatment *
(N = 254) n (%) | Control †
(N = 146) n (%) |
| Values represent patient counts and percentages. A patient reporting more than one adverse event for a particular MedDRA preferred term is counted only once. | ||
|
||
| Asthma | 12 (4.7) | 6 (4.1) |
| Headache | 9 (3.5) | 5 (3.4) |
| Epistaxis | 6 (2.4) | 2 (1.4) |
| Presyncope | 6 (2.4) | 3 (2.1) |
| Bronchitis | 5 (2.0) | 2 (1.4) |
| Otitis media | 5 (2.0) | 2 (1.4) |
| Nasopharyngitis | 3 (1.2) | 1 (0.7) |
Study 1 monitored patients from Day 90 through 6 months. Hypersensitivity (4% (n=2) vs. 0), chronic rhinosinusitis (11% (n=6) vs. 9% (n=4)), and upper respiratory tract infections (8% (n=4) vs. 2% (n=1)) were reported in more than 2 subjects in the treatment group, and more commonly than the control group during this time period.
The safety of repeat administration of the SINUVA Sinus Implant was evaluated in Study 3 that was an open-label, single-arm, multicenter study in 50 patients. All patients underwent an in- office bilateral placement of the SINUVA Sinus Implant in each ethmoid sinus (totaling 2 implants) and were followed for 365 days. Patients were required to use mometasone furoate nasal spray once daily (200 mcg of mometasone furoate) through 365 days. At 90 days, the remaining implants were removed. To maximize the size of the safety population, patients with ethmoid sinus polyps grade ≥ 1 on any side were considered for repeat implant placement. Repeat placement was not performed if polyp grade was < 1, or if the patient declined it. Of the 50 patients, 41 received repeat implant placement (33 bilaterally and 8 unilaterally). Acute sinusitis (29%, n=12), upper respiratory infection (17%, n=7) epistaxis (12%, n=5), nasal discomfort or rhinalgia (12%, n=5), headache (7%, n=3), were the common adverse reactions that occurred in at least 3 subjects who underwent repeat placement during the study period.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of the SINUVA sinus implant. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to SINUVA, or a combination of these factors, include: implant migration, lack of efficacy, nasal pain, headache, epistaxis. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug experience.
7. Drug Interactions
Formal drug-drug interaction studies have not been conducted with the SINUVA Sinus Implant. An evaluation of the concurrent administration of the SINUVA Sinus Implant and other commonly used nasal drugs was not associated with any unusual adverse reactions.
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of the SINUVA Sinus Implant have not been established in pediatric patients less than 18 years of age.
10. Overdosage
There are no data available on the effects of acute or chronic overdosage with SINUVA Sinus Implant. Chronic overdosage may result in signs/symptoms of hypercorticism [see Warnings and Precautions (5.5)].
11. Sinuva Description
The SINUVA Sinus Implant is a self-expanding, bioabsorbable, drug eluting implant provided with a crimper and a single-use delivery system. SINUVA Sinus Implant is comprised of poly(L-lactide-co-glycolide) and poly(L-lactide-co-⃞-caprolactone) coated with mometasone furoate embedded in a bioabsorbable polymer matrix containing poly(DL-lactide-co-glycolide) and polyethylene glycol (inactive ingredients) which provides for gradual release of the drug. The SINUVA Sinus Implant is packaged in a tray, which is then sealed in a foil pouch and placed in the product carton. The SINUVA Sinus Implant is provided sterile.
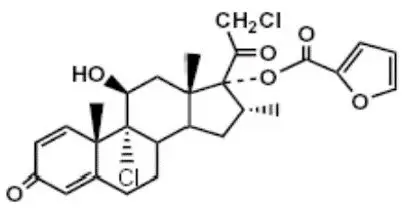
Mometasone furoate, the active component of the SINUVA Sinus Implant, is a corticosteroid with the chemical name 9,21-dichloro-11(⃞),17-dihydroxy-16(⃞)-methylpregna-1,4-diene-3,20- dione 17 (2-furoate). Mometasone furoate is a white powder with an empirical formula of C27H30Cl2O6, and molecular weight of 521.44 Daltons.
The chemical structure of mometasone furoate is shown below:
The inactive ingredients are poly-(DL-lactide-co-glycolide) and polyethylene glycol. Poly-(DL-lactide-co-glycolide) is an amorphous biodegradable polymer.
12. Sinuva - Clinical Pharmacology
12.1 Mechanism of Action
Mometasone furoate is a corticosteroid demonstrating potent anti-inflammatory activity. The precise mechanism of corticosteroid action on inflammation is not known. Corticosteroids have been shown to have a wide range of effects on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in inflammation.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year carcinogenicity study in Sprague Dawley rats, mometasone furoate demonstrated no statistically significant increase of tumors at inhalation doses up to 67 mcg/kg (approximately 14 times the MRHD on an AUC basis). In a 19-month carcinogenicity study in Swiss CD-1 mice, mometasone furoate demonstrated no statistically significant increase in the incidence of tumors at inhalation doses up to 160 mcg/kg (approximately 9 times the MRHD on an AUC basis).
Mometasone furoate increased chromosomal aberrations in an in vitro Chinese hamster ovary cell assay, but did not have this effect in an in vitro Chinese hamster lung cell assay. Mometasone furoate was not mutagenic in the Ames test or mouse lymphoma assay, and was not clastogenic in an in vivo mouse micronucleus assay, a rat bone marrow chromosomal aberration assay, or a mouse male germ-cell chromosomal aberration assay. Mometasone furoate also did not induce unscheduled DNA synthesis in vivo in rat hepatocytes.
In reproductive studies in rats, impairment of fertility was not produced by subcutaneous doses up to 15 mcg/kg (approximately 8 times the MRHD on an AUC basis).
14. Clinical Studies
The SINUVA Sinus Implant was evaluated in 450 patients, 18 years of age and older, with chronic rhinosinusitis with nasal polyps and a history of ethmoid sinus surgery. The development program included a trial of 6 months duration (Study 1: NCT01732536), another trial of 90 days duration (Study 2: NCT02291549), and a repeat placement study of 1-year duration (Study 3: NCT03358329) [see Adverse Reactions (6.1)]. The efficacy of SINUVA Sinus Implant is based primarily on Study 2 as described below.
Study 2 was a randomized, controlled, single-blind, multicenter (all sites were in the US) study with 300 patients: 201 patients were assigned to the treatment group and underwent bilateral placement of the SINUVA Sinus Implants in the ethmoid sinuses. The remaining 99 patients were assigned to the control group and underwent a placebo (sham) procedure, consisting of advancement of the Delivery System with the SINUVA Sinus Implant into the ethmoid sinuses, followed by removal of the Delivery System without deployment of the SINUVA Sinus Implant. The Implants were removed by Day 60 to allow blinded grading at Day 90. All patients [treatment (T) and control (C) groups] were required to use a mometasone furoate nasal spray once daily (200 mcg of mometasone furoate) through Day 90.
The co-primary efficacy endpoints were:
- Change from baseline to Day 30 in Nasal Obstruction/Congestion score, as determined by patients using a daily diary; and
- Change from baseline to Day 90 in bilateral polyp grade, as determined from video- endoscopies reviewed by an independent panel of 3 sinus surgeons who were masked to treatment assignment.
The study population consisted of adult patients (≥ 18 years of age) diagnosed with chronic rhinosinusitis who had undergone prior bilateral total ethmoidectomy but were indicated for revision endoscopic sinus surgery because they presented with recurrent nasal obstruction/congestion symptoms and recurrent bilateral sinus obstruction due to nasal polyps. Subjects were excluded for other grade 3 or 4 adhesions/synechiae, grade 4 polyps, acute bacterial or invasive fungal sinusitis, and immune deficiency, including cystic fibrosis. There were no statistically significant differences between groups in baseline demographics and clinical characteristics, except the treatment group had a higher proportion of asthma patients (T: 74% vs. C: 62%) and higher mean Percent Ethmoid Sinus Obstruction score [T: 76 (SD 17.4) vs. C: 69 (SD 19.9)]. The random imbalances did not impact treatment effect.
The co-primary efficacy results are presented in Table 2. The treatment group demonstrated a statistically significant difference from baseline to Day 30 in Nasal Obstruction/Congestion score and from baseline to Day 90 in bilateral polyp grade, compared to the control group.
| Treatment (N = 201) | Control (N = 99) |
||
|---|---|---|---|
|
|||
| Nasal Obstruction/Congestion Score* | |||
| N | 201 | 99 | |
| Baseline, Mean (SD) | 2.36 (0.49) | 2.35 (0.48) | |
| Change from Baseline, Mean (SD) | -0.80 (0.73) | -0.56 (0.62) | |
| Difference vs. Control (95% CI)† | -0.23 (-0.39, -0.06) | ||
| Bilateral Polyp Grade‡ | |||
| N | 195 | 97 | |
| Baseline, Mean (SD) | 5.48 (1.13) | 5.43 (1.01) | |
| Change from Baseline, Mean (SD) | -0.56 (1.06) | -0.15 (0.91) | |
| Difference vs. Control (95% CI)† | -0.35 (-0.60, -0.09) | ||
Change from baseline to Day 90 in the mean Percent Ethmoid Sinus Obstruction score (100 mm VAS), as judged by the independent panel [Difference vs. control: -7.96%; 95% CI (-12.1, -3.8)], met statistical significance and supported the co-primary endpoints.
16. How is Sinuva supplied
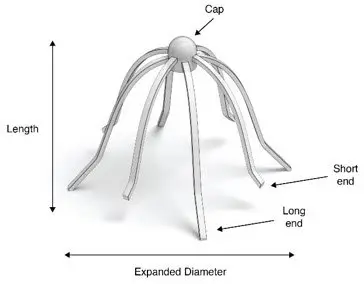
A carton of SINUVA contains one SINUVA (1350 mcg mometasone furoate) implant inside of a Crimper and one disposable Delivery System packaged in a foil pouch. The SINUVA Sinus Implant is 20 mm in length and 34 mm in expanded diameter (Figure 1).
17. Patient Counseling Information
- Encourage patients to use saline irrigations or sprays regularly.
- Advise the patient that the Implant is bioabsorbable and intended to soften over time. As the Implant softens and polyps decrease, the Implant may be expelled out of the nose on its own or with actions such as sneezing or forceful nose blowing.
- Advise the patient to call a health care professional immediately if they experience any of the following:
- Excessive nasal bleeding or symptoms of infection, such as excessive pain or discomfort, persistent headache, increased sinus discharge.
- Symptoms suggesting the Implant has migrated posteriorly, such as irritation or choking sensation in the back of the throat or swallowing the Implant.
| SINUVA
mometasone furoate implant |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Intersect ENT, Inc. (876715355) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Intersect ENT, Inc. | 876715355 | MANUFACTURE(10599-003) | |