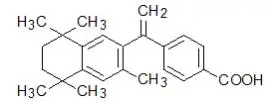
Drug Detail:Targretin (topical) (Bexarotene (topical) [ bex-ar-oh-teen ])
Drug Class: Miscellaneous topical agents
Targretin Gel - Clinical Pharmacology
Contraindications
Targretin® gel 1% is contraindicated in patients with a known hypersensitivity to bexarotene or other components of the product.
Pregnancy: Category X
Targretin® gel 1% may cause fetal harm when administered to a pregnant woman.
Targretin® gel must not be given to a pregnant woman or a woman who intends to become pregnant. If a woman becomes pregnant while taking Targretin® gel, Targretin® gel must be stopped immediately and the woman given appropriate counseling.
Bexarotene caused malformations when administered orally to pregnant rats during days 7-17 of gestation. Developmental abnormalities included incomplete ossification at 4 mg/kg/day and cleft palate, depressed eye bulge/microphthalmia, and small ears at 16 mg/kg/day. At doses greater than 10 mg/kg/day, bexarotene caused developmental mortality. The no-effect oral dose in rats was 1 mg/kg/day. Plasma bexarotene concentrations in patients with CTCL applying Targretin® gel 1% were generally less than one hundredth the Cmax associated with dysmorphogenesis in rats, although some patients had Cmax levels that were approximately one eighth the concentration associated with dysmorphogenesis in rats.
Women of child-bearing potential should be advised to avoid becoming pregnant when Targretin® gel is used. The possibility that a woman of child-bearing potential is pregnant at the time therapy is instituted should be considered. A negative pregnancy test (e.g., serum beta-human chorionic gonadotropin, beta-HCG) with a sensitivity of at least 50 mIU/L should be obtained within one week prior to Targretin® gel therapy, and the pregnancy test must be repeated at monthly intervals while the patient remains on Targretin® gel. Effective contraception must be used for one month prior to the initiation of therapy, during therapy and for at least one month following discontinuation of therapy; it is recommended that two reliable forms of contraception be used simultaneously unless abstinence is the chosen method. Male patients with sexual partners who are pregnant, possibly pregnant, or who could become pregnant must use condoms during sexual intercourse while applying Targretin® gel and for at least one month after the last dose of drug. Targretin® gel therapy should be initiated on the second or third day of a normal menstrual period. No more than a one month supply of Targretin® gel should be given to the patient so that the results of pregnancy testing can be assessed and counseling regarding avoidance of pregnancy and birth defects can be reinforced.
Precautions
Pregnancy: Category X. See CONTRAINDICATIONS
General: Targretin® gel should be used with caution in patients with a known hypersensitivity to other retinoids. No clinical instances of cross-reactivity have been noted.
Vitamin A Supplementation: In clinical studies, patients were advised to limit vitamin A intake to ≤15,000 IU/day. Because of the relationship of bexarotene to vitamin A, patients should be advised to limit vitamin A supplements to avoid potential additive toxic effects.
Photosensitivity: Retinoids as a class have been associated with photosensitivity. In vitro assays indicate that bexarotene is a potential photosensitizing agent. There were no reports of photosensitivity in patients in the clinical studies. Patients should be advised to minimize exposure to sunlight and artificial ultraviolet light during the use of Targretin® gel.
Renal Insufficiency
No formal studies have been conducted with Targretin® gel in patients with renal insufficiency. Urinary elimination of bexarotene and its known metabolites is a minor excretory pathway for bexarotene (<1% of an orally administered dose), but because renal insufficiency can result in significant protein binding changes, and bexarotene is >99% protein bound, pharmacokinetics may be altered in patients with renal insufficiency.
Hepatic Insufficiency
No specific studies have been conducted with Targretin® gel in patients with hepatic insufficiency. Because less than 1% of the dose of oral bexarotene is excreted in the urine unchanged and there is in vitro evidence of extensive hepatic contribution to bexarotene elimination, hepatic impairment would be expected to lead to greatly decreased clearance.
| TARGRETIN
bexarotene gel |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Eisai Inc. (831600833) |