Drug Detail:Tascenso odt (Fingolimod)
Drug Class: Selective immunosuppressants
Highlights of Prescribing Information
TASCENSO ODT (fingolimod) orally disintegrating tablets
Initial U.S. Approval: 2010
Recent Major Changes
Indications and Usage (1) 12/2022
Dosage and Administration (2.2, 2.3, 2.4) 12/2022
Warnings and Precautions (5.1, 5.2) 12/2022
Warnings and Precautions (5.3, 5.9) 8/2023
Indications and Usage for Tascenso ODT
TASCENSO ODT is a sphingosine 1-phosphate receptor modulator indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in patients 10 years of age and older. (1)
Tascenso ODT Dosage and Administration
- Assessments are required prior to initiating TASCENSO ODT (2.1)
- Recommended dosage for adults and pediatric patients (10 years of age and older) weighing more than 40 kg: 0.5 mg orally once daily, with or without food. (2.2, 2.3)
- Recommended dosage for pediatric patients (10 years of age and older) weighing less than or equal to 40 kg: 0.25 mg orally once daily, with or without food (2.2, 2.3).
- Administer TASCENSO ODT with or without water. Place tablet directly on the tongue and allow it to dissolve before swallowing. (2.2)
- First-Dose Monitoring (including reinitiation after discontinuation greater than 14 days and dose increases):
- Observe all patients for bradycardia for at least 6 hours; monitor pulse and blood pressure hourly. Electrocardiograms (ECGs) prior to dosing and at end of observation period required. (2.4)
- Monitor until resolution if heart rate < 45 beats per minute (bpm) in adults, < 55 bpm in patients aged 12 years and above, or < 60 bpm in pediatric patients aged 10 to below 12 years, atrioventricular (AV) block, or if lowest postdose heart rate is at the end of the observation period. (2.4)
- Monitor symptomatic bradycardia with ECG until resolved. Continue overnight if intervention is required; repeat first-dose monitoring for second dose. (2.4)
- Observe patients overnight if at higher risk of symptomatic bradycardia, heart block, prolonged QTc interval, or if taking drugs with known risk of torsades de pointes. (2.4, 7.1)
Dosage Forms and Strengths
Orally disintegrating tablets: 0.25 mg and 0.5 mg (3)
Contraindications
- Recent myocardial infarction, unstable angina, stroke, transient ischemic attack, decompensated heart failure with hospitalization, or Class III/IV heart failure. (4)
- History of Mobitz Type II 2nd degree or 3rd degree AV block or sick sinus syndrome, unless patient has a pacemaker. (4)
- Baseline QTc interval ≥ 500 msec. (4)
- Cardiac arrhythmias requiring anti-arrhythmic treatment with Class Ia or Class III anti-arrhythmic drugs. (4)
- Hypersensitivity to fingolimod or its excipients. (4)
- Concomitant use with other products containing fingolimod. (4)
Warnings and Precautions
- Bradyarrhythmia and Atrioventricular Blocks: Because of a risk for bradyarrhythmia and AV blocks, monitor during initiation of treatment (2.4, 5.1)
- Infections: TASCENSO ODT may increase the risk. Obtain a complete blood count (CBC) before initiating TASCENSO ODT (i.e., within 6 months). Monitor for infection during treatment and for 2 months after discontinuation. Do not start in patients with active infections. (5.2)
- Progressive Multifocal Leukoencephalopathy (PML): Withhold TASCENSO ODT at the first sign or symptom suggestive of PML. (5.3)
- Macular Edema: Examine the fundus before and 3-4 months after treatment start. Diabetes mellitus and uveitis increase the risk. (5.4)
- Liver Injury: Obtain liver enzyme results before initiation and periodically during treatment. Closely monitor patients with severe hepatic impairment. Discontinue if there is evidence of liver injury without other cause. (5.5, 8.6, 12.3)
- Posterior Reversible Encephalopathy Syndrome (PRES): If suspected, discontinue TASCENSO ODT. (5.6)
- Respiratory Effects: Evaluate when clinically indicated. (5.7)
- Fetal Risk: May cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use an effective method of contraception during treatment and for 2 months after stopping TASCENSO ODT. (5.8, 8.1, 8.3)
- Severe Increase in Disability After Stopping TASCENSO ODT: Monitor for development of severe increase in disability following discontinuation and begin appropriate treatment as needed. (5.9)
- Tumefactive MS: Consider when severe MS relapse occurs during treatment or after discontinuation. Obtain imaging and begin treatment as needed. (5.10)
- Increased Blood Pressure (BP): Monitor BP during treatment. (5.11)
- Malignancies: Suspicious skin lesions should be evaluated. (5.12)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥ 10% and greater than placebo): Headache, liver transaminase elevation, diarrhea, cough, influenza, sinusitis, back pain, abdominal pain, and pain in extremity. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Cycle Pharmaceuticals Ltd at 1-888-533-1625 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Systemic Ketoconazole: Monitor during concomitant use. (7.2, 12.3)
- Vaccines: Avoid live attenuated vaccines during, and for 2 months after stopping TASCENSO ODT treatment. (5.3, 7.3)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2023
Full Prescribing Information
1. Indications and Usage for Tascenso ODT
TASCENSO ODT is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in patients 10 years of age and older.
2. Tascenso ODT Dosage and Administration
2.2 Important Administration Instructions
Patients who initiate TASCENSO ODT, and those who reinitiate treatment after discontinuation for longer than 14 days, require first-dose monitoring. This monitoring is also recommended when the dose is increased in pediatric patients. Patients who are currently treated with another fingolimod product and underwent first-dose monitoring at initiation may be switched to TASCENSO ODT at the same daily dose without a need to repeat first-dose monitoring (unless the previous treatment was discontinued more than 14 days prior) [see Dosage and Administration (2.4, 2.5)].
TASCENSO ODT can be taken with or without food.
Inform the patient about the following administration instructions for TASCENSO ODT:
- Use dry hands when opening the blister pack.
- Peel back the foil covering of one blister and gently remove the orally disintegrating tablet (ODT). Do not push the ODT through the foil.
- As soon as the blister is opened, remove the ODT and place on the tongue and allow it to dissolve before swallowing.
- The ODT may be taken with or without water.
- Take the ODT immediately after opening the blister pack. Do not store the ODT outside the blister pack for future use.
2.3 Recommended Dosage
In adults and pediatric patients 10 years of age and older weighing more than 40 kg, the recommended dosage of TASCENSO ODT is
0.5 mg orally once-daily.
In pediatric patients 10 years of age and older weighing less than or equal to 40 kg, the recommended dosage of TASCENSO ODT is
0.25 mg orally once daily.
Fingolimod doses higher than 0.5 mg are associated with a greater incidence of adverse reactions without additional benefit.
2.4 First-Dose Monitoring
Initiation of TASCENSO ODT treatment results in a decrease in heart rate, for which monitoring is recommended [see Warnings and Precautions (5.1), Clinical Pharmacology (12.2)]. Prior to dosing and at the end of the observation period, obtain an electrocardiogram (ECG) in all patients.
2.5 Monitoring After Reinitiation of Therapy Following Discontinuation
When restarting TASCENSO ODT treatment after discontinuation for more than 14 days after the first month of treatment, perform first-dose monitoring, because effects on heart rate and AV conduction may recur on reintroduction of TASCENSO ODT treatment [see Dosage and Administration (2.4)]. The same precautions (first-dose monitoring) as for initial dosing are applicable. Within the first 2 weeks of treatment, first-dose procedures are recommended after interruption of 1 day or more; during Weeks 3 and 4 of treatment, first-dose procedures are recommended after treatment interruption of more than 7 days.
3. Dosage Forms and Strengths
TASCENSO ODT is available as:
-
0.25 mg orally disintegrating tablets are white to off white, round tablets debossed with
 .
. -
0.5 mg orally disintegrating tablets are white to off white, round tablets debossed with
 .
.
4. Contraindications
TASCENSO ODT is contraindicated in patients who have:
- in the last 6 months experienced myocardial infarction, unstable angina, stroke, TIA, decompensated heart failure requiring hospitalization or Class III/IV heart failure
- a history or presence of Mobitz Type II second-degree or third-degree AV block or sick sinus syndrome, unless patient has a functioning pacemaker [see Warnings and Precautions (5.1)]
- a baseline QTc interval ≥ 500 msec
- cardiac arrhythmias requiring anti-arrhythmic treatment with Class Ia or Class III anti-arrhythmic drugs
- had a hypersensitivity reaction to fingolimod or any of the excipients in TASCENSO ODT. Observed reactions include rash, urticaria, and angioedema [see Warnings and Precautions (5.14)].
- Concomitant use with other products containing fingolimod
5. Warnings and Precautions
5.1 Bradyarrhythmia and Atrioventricular Blocks
Because of a risk for bradyarrhythmia and AV blocks, patients should be monitored during TASCENSO ODT treatment initiation [see Dosage and Administration (2.4)].
5.3 Progressive Multifocal Leukoencephalopathy
Cases of progressive multifocal leukoencephalopathy (PML) have occurred in patients with MS who received fingolimod, the active moiety in TASCENSO ODT, in the postmarketing setting. PML is an opportunistic viral infection of the brain caused by the JC virus (JCV) that typically only occurs in patients who are immunocompromised, and that usually leads to death or severe disability. PML has occurred in patients who had not been treated previously with natalizumab, which has a known association with PML, were not taking any other immunosuppressive or immunomodulatory medications concomitantly and did not have any ongoing systemic medical conditions resulting in compromised immune system function. The majority of cases have occurred in patients treated with fingolimod for at least 2 years. The relationship between the risk of PML and the duration of treatment is unknown.
At the first sign or symptom suggestive of PML, withhold TASCENSO ODT and perform an appropriate diagnostic evaluation. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes.
MRI findings may be apparent before clinical signs or symptoms. Cases of PML, diagnosed based on MRI findings and the detection of JCV DNA in the cerebrospinal fluid in the absence of clinical signs or symptoms specific to PML, have been reported in patients treated with MS medications associated with PML, including fingolimod. Many of these patients subsequently became symptomatic with PML. Therefore, monitoring with MRI for signs that may be consistent with PML may be useful, and any suspicious findings should lead to further investigation to allow for an early diagnosis of PML, if present. Lower PML-related mortality and morbidity have been reported following discontinuation of another MS medication associated with PML in patients with PML who were initially asymptomatic compared to patients with PML who had characteristic clinical signs and symptoms at diagnosis. It is not known whether these differences are due to early detection and discontinuation of MS treatment or due to differences in disease in these patients.
If PML is confirmed, treatment with TASCENSO ODT should be discontinued.
Immune reconstitution inflammatory syndrome (IRIS) has been reported in patients treated with S1P receptor modulators, including fingolimod, who developed PML and subsequently discontinued treatment. IRIS presents as a clinical decline in the patient’s condition that may be rapid, can lead to serious neurological complications or death, and is often associated with characteristic changes on MRI. The time to onset of IRIS in patients with PML was generally within a few months after S1P receptor modulator discontinuation. Monitoring for development of IRIS and appropriate treatment of the associated inflammation should be undertaken.
5.4 Macular Edema
Sphingosine 1-phosphate (S1P) receptor modulators, including TASCENSO ODT, have been associated with an increased risk of macular edema. Perform an examination of the fundus, including the macula, in all patients before starting treatment, again 3 to 4 months after starting treatment, and again at any time after a patient reports visual disturbances while on TASCENSO ODT therapy.
A dose-dependent increase in the risk of macular edema occurred in the fingolimod capsules clinical development program.
In 2-year double-blind, placebo-controlled studies in adult patients with multiple sclerosis, macular edema with or without visual symptoms occurred in 1.5% of patients (11/799) treated with fingolimod 1.25 mg capsules, 0.5% of patients (4/783) treated with fingolimod 0.5 mg capsules, and 0.4% of patients (3/773) treated with placebo. Macular edema occurred predominantly during the first 3 to 4 months of therapy. These clinical trials excluded patients with diabetes mellitus, a known risk factor for macular edema (see below Macular Edema in Patients with History of Uveitis or Diabetes Mellitus). Symptoms of macular edema included blurred vision and decreased visual acuity. Routine ophthalmological examination detected macular edema in some patients with no visual symptoms. Macular edema generally partially or completely resolved with or without treatment after drug discontinuation. Some patients had residual visual acuity loss even after resolution of macular edema. Macular edema has also been reported in patients taking fingolimod in the postmarketing setting, usually within the first 6 months of treatment.
Continuation of TASCENSO ODT in patients who develop macular edema has not been evaluated. A decision on whether or not to discontinue TASCENSO ODT therapy should include an assessment of the potential benefits and risks for the individual patient. The risk of recurrence after rechallenge has not been evaluated.
Macular Edema in Patients with History of Uveitis or Diabetes Mellitus
Patients with a history of uveitis and patients with diabetes mellitus are at increased risk of macular edema during TASCENSO ODT therapy. The incidence of macular edema is also increased in MS patients with a history of uveitis. In the combined clinical trial experience in adult patients with all doses of fingolimod capsules, the rate of macular edema was approximately 20% in MS patients with a history of uveitis versus 0.6% in those without a history of uveitis. Fingolimod, the active moiety in TASCENSO ODT, has not been tested in MS patients with diabetes mellitus. In addition to the examination of the fundus including the macula prior to treatment and at 3 to 4 months after starting treatment, MS patients with diabetes mellitus or a history of uveitis should have regular follow-up examinations.
5.5 Liver Injury
Clinically significant liver injury has occurred in patients treated with fingolimod in the postmarketing setting. Signs of liver injury, including markedly elevated serum hepatic enzymes and elevated total bilirubin, have occurred as early as ten days after the first dose and have also been reported after prolonged use. Cases of acute liver failure requiring liver transplant have been reported.
In 2-year placebo-controlled clinical trials in adult patients, elevation of liver enzymes (ALT, AST and GGT) to 3-fold the upper limit of normal (ULN) or greater occurred in 14% of patients treated with fingolimod 0.5 mg capsules and 3% of patients on placebo. Elevations 5-fold the ULN or greater occurred in 4.5% of patients on fingolimod capsules and 1% of patients on placebo. The majority of elevations occurred within 6 to 9 months. In clinical trials, fingolimod capsules were discontinued if the elevation exceeded 5 times the ULN. Serum transaminase levels returned to normal within approximately 2 months after discontinuation of fingolimod capsules. Recurrence of liver transaminase elevations occurred with rechallenge in some patients.
Prior to starting treatment with TASCENSO ODT (within 6 months), obtain serum transaminases (ALT and AST) and total bilirubin levels. Obtain transaminase levels and total bilirubin levels periodically until two months after TASCENSO ODT discontinuation.
Patients should be monitored for signs and symptoms of any hepatic injury. Measure liver transaminase and bilirubin levels promptly in patients who report symptoms that may indicate liver injury, including new or worsening fatigue, anorexia, right upper abdominal discomfort, dark urine, or jaundice. In this clinical context, if the patient is found to have an alanine aminotransferase (ALT) greater than three times the reference range with serum total bilirubin greater than two times the reference range, treatment with TASCENSO ODT treatment should be interrupted. Treatment should not be resumed if a plausible alternative etiology for the signs and symptoms cannot be established, because these patients are at risk for severe drug-induced liver injury.
Because fingolimod exposure is doubled in patients with severe hepatic impairment, these patients should be closely monitored during treatment with TASCENSO ODT, as the risk of adverse reactions is greater [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
5.6 Posterior Reversible Encephalopathy Syndrome
There have been rare cases of posterior reversible encephalopathy syndrome (PRES) reported in adult patients receiving fingolimod. Symptoms reported included sudden onset of severe headache, altered mental status, visual disturbances, and seizure. Symptoms of PRES are usually reversible but may evolve into ischemic stroke or cerebral hemorrhage. Delay in diagnosis and treatment may lead to permanent neurological sequelae. If PRES is suspected, TASCENSO ODT should be discontinued.
5.7 Respiratory Effects
Dose-dependent reductions in forced expiratory volume over 1 second (FEV1) and diffusion lung capacity for carbon monoxide (DLCO) were observed in patients treated with fingolimod, the active moiety in TASCENSO ODT, as early as 1 month after treatment initiation. In 2-year placebo-controlled trials in adult patients, the reduction from baseline in the percent of predicted values for FEV1 at the time of last assessment on drug was 2.8% for fingolimod 0.5 mg capsules and 1.0% for placebo. For DLCO, the reduction from baseline in percent of predicted values at the time of last assessment on drug was 3.3% for fingolimod 0.5 mg capsules and 0.5% for placebo. The changes in FEV1 appear to be reversible after treatment discontinuation. There is insufficient information to determine the reversibility of the decrease of DLCO after drug discontinuation. In MS placebo-controlled trials in adult patients, dyspnea was reported in 9% of patients receiving fingolimod 0.5 mg capsules and 7% of patients receiving placebo. Several patients discontinued fingolimod capsules because of unexplained dyspnea during the extension (uncontrolled) studies. Fingolimod, the active moiety in TASCENSO ODT, has not been tested in MS patients with compromised respiratory function.
Spirometric evaluation of respiratory function and evaluation of DLCO should be performed during therapy with TASCENSO ODT if clinically indicated.
5.8 Fetal Risk
Based on findings from animal studies, TASCENSO ODT may cause fetal harm when administered to a pregnant woman. In animal reproduction studies conducted in rats and rabbits, developmental toxicity was observed with administration of fingolimod at doses less than the recommended human dose. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Because it takes approximately 2 months to eliminate fingolimod from the body, advise females of reproductive potential to use effective contraception to avoid pregnancy during and for 2 months after stopping TASCENSO ODT treatment [see Use in Specific Populations (8.1, 8.3)].
5.9 Severe Increase in Disability After Stopping TASCENSO ODT
Severe increase in disability accompanied by multiple new lesions on MRI has been reported after discontinuation of fingolimod in the postmarketing setting. Patients in most of these reported cases did not return to the functional status they had before stopping fingolimod. The increase in disability generally occurred within 12 weeks after stopping fingolimod but was reported up to 24 weeks after fingolimod discontinuation.
Monitor patients for development of severe increase in disability following discontinuation of TASCENSO ODT and begin appropriate treatment as needed.
After stopping TASCENSO ODT in the setting of PML, monitor for development of immune reconstitution inflammatory syndrome (PML-IRIS) [see Warnings and Precautions (5.3)].
5.10 Tumefactive Multiple Sclerosis
MS relapses with tumefactive demyelinating lesions on imaging have been observed during fingolimod therapy and after fingolimod discontinuation in the postmarketing setting. Most reported cases of tumefactive MS in patients receiving fingolimod have occurred within the first 9 months after fingolimod initiation, but tumefactive MS may occur at any point during treatment. Cases of tumefactive MS have also been reported within the first 4 months after fingolimod discontinuation.
Tumefactive MS should be considered when a severe MS relapse occurs during TASCENSO ODT treatment, especially during initiation, or after discontinuation of TASCENSO ODT, prompting imaging evaluation and initiation of appropriate treatment.
5.11 Increased Blood Pressure
In adult MS controlled clinical trials, patients treated with fingolimod 0.5 mg capsules had an average increase over placebo of approximately 3 mmHg in systolic pressure, and approximately 2 mmHg in diastolic pressure, first detected after approximately 1 month of fingolimod treatment initiation and persisting with continued treatment. Hypertension was reported as an adverse reaction in 8% of patients on fingolimod 0.5 mg capsules and in 4% of patients on placebo. Blood pressure should be monitored during treatment with TASCENSO ODT.
5.13 Immune System Effects Following TASCENSO ODT Discontinuation
Fingolimod remains in the blood and has pharmacodynamic effects, including decreased lymphocyte counts, for up to 2 months following the last dose of TASCENSO ODT. Lymphocyte counts generally return to the normal range within 1-2 months of stopping therapy [see Clinical Pharmacology (12.2)]. Because of the continuing pharmacodynamic effects of fingolimod, initiating other drugs during this period warrants the same considerations needed for concomitant administration (e.g., risk of additive immunosuppressant effects) [see Drug Interactions (7.4)].
5.14 Hypersensitivity Reactions
Hypersensitivity reactions, including rash, urticaria, and angioedema have been reported with fingolimod in the postmarketing setting. TASCENSO ODT is contraindicated in patients with history of hypersensitivity to fingolimod or any of its excipients [see Contraindications (4)].
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described elsewhere in labeling:
- Bradyarrhythmia and Atrioventricular Blocks [see Warnings and Precautions (5.1)]
- Infections [see Warnings and Precautions (5.2)]
- Progressive Multifocal Leukoencephalopathy [see Warnings and Precautions (5.3)]
- Macular Edema [see Warnings and Precautions (5.4)]
- Liver Injury [see Warnings and Precautions (5.5)]
- Posterior Reversible Encephalopathy Syndrome [see Warnings and Precautions (5.6)]
- Respiratory Effects [see Warnings and Precautions (5.7)]
- Fetal Risk [see Warnings and Precautions (5.8)]
- Severe Increase in Disability After Stopping TASCENSO ODT [see Warnings and Precautions (5.9)]
- Tumefactive Multiple Sclerosis [see Warnings and Precautions (5.10)]
- Increased Blood Pressure [see Warnings and Precautions (5.11)]
- Malignancies [see Warnings and Precautions (5.12)]
- Immune System Effects Following TASCENSO ODT Discontinuation [see Warnings and Precautions (5.13)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.14)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of fingolimod. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: Hemolytic anemia and thrombocytopenia
Hepatobiliary Disorders: Liver injury [see Warnings and Precautions (5.5)]
Infections: infections including cryptococcal infections [see Warnings and Precautions (5.2)], human papilloma virus (HPV) infection, including papilloma, dysplasia, warts and HPV-related cancer [see Warnings and Precautions (5.2)], progressive multifocal leukoencephalopathy [see Warnings and Precautions (5.3)]
Musculoskeletal and connective tissue disorders: arthralgia, myalgia
Nervous system disorders: posterior reversible encephalopathy syndrome [see Warnings and Precautions (5.6)], seizures, including status epilepticus [see Adverse Reactions (6.1)]
Neoplasms, benign, malignant, and unspecified (including cysts and polyps): melanoma, Merkel cell carcinoma, and cutaneous T-cell lymphoma (including mycosis fungoides) [see Warnings and Precautions (5.12)]
Skin and subcutaneous tissue disorders: hypersensitivity [see Warnings and Precautions (5.14)]
7. Drug Interactions
7.1 QT Prolonging Drugs
TASCENSO ODT has not been studied in patients treated with drugs that prolong the QT interval. Drugs that prolong the QT interval have been associated with cases of torsades de pointes in patients with bradycardia. Since initiation of TASCENSO ODT treatment results in decreased heart rate and may prolong the QT interval, patients on QT prolonging drugs with a known risk of torsades de pointes (e.g., citalopram, chlorpromazine, haloperidol, methadone, erythromycin) should be monitored overnight with continuous ECG in a medical facility [see Dosage and Administration (2.4), Warnings and Precautions (5.1)].
7.2 Ketoconazole
The blood levels of fingolimod and fingolimod-phosphate are increased by 1.7-fold when used concomitantly with ketoconazole. Patients who use TASCENSO ODT and systemic ketoconazole concomitantly should be closely monitored, as the risk of adverse reactions is greater.
7.3 Vaccines
TASCENSO ODT reduces the immune response to vaccination. Vaccination may be less effective during and for up to 2 months after discontinuation of treatment with TASCENSO ODT [see Clinical Pharmacology (12.2)]. Avoid the use of live attenuated vaccines during and for 2 months after treatment with TASCENSO ODT because of the risk of infection.
It is recommended that pediatric patients, if possible, be brought up to date with all immunizations in agreement with current immunization guidelines prior to initiating TASCENSO ODT therapy.
7.4 Antineoplastic, Immunosuppressive, or Immune-Modulating Therapies
Antineoplastic, immune-modulating, or immunosuppressive therapies, (including corticosteroids) are expected to increase the risk of immunosuppression, and the risk of additive immune system effects must be considered if these therapies are coadministered with TASCENSO ODT. When switching from drugs with prolonged immune effects, such as natalizumab, teriflunomide or mitoxantrone, the duration and mode of action of these drugs must be considered to avoid unintended additive immunosuppressive effects when initiating TASCENSO ODT [see Warnings and Precautions (5.2)].
7.5 Drugs That Slow Heart Rate or Atrioventricular Conduction (e.g., beta blockers or diltiazem)
Experience with fingolimod in patients receiving concurrent therapy with drugs that slow the heart rate or AV conduction (e.g., beta blockers, digoxin, or heart rate-slowing calcium channel blockers such as diltiazem or verapamil) is limited. Because initiation of TASCENSO ODT treatment may result in an additional decrease in heart rate, concomitant use of these drugs during TASCENSO ODT initiation may be associated with severe bradycardia or heart block. Seek advice from the physician prescribing these drugs regarding the possibility to switch to drugs that do not slow the heart rate or atrioventricular conduction before initiating TASCENSO ODT. Patients who cannot switch should have overnight continuous ECG monitoring after the first dose [see Dosage and Administration (2.4), Warnings and Precautions (5.1)].
7.6 Laboratory Test Interaction
Because fingolimod reduces blood lymphocyte counts via redistribution in secondary lymphoid organs, peripheral blood lymphocyte counts cannot be utilized to evaluate the lymphocyte subset status of a patient treated with fingolimod. A recent CBC should be available before initiating treatment with TASCENSO ODT.
8. Use In Specific Populations
8.4 Pediatric Use
Safety and effectiveness of fingolimod for the treatment of relapsing forms of multiple sclerosis in pediatric patients 10 to less than 18 years of age were established in one randomized, double-blind clinical study in 215 patients (fingolimod n = 107; intramuscular interferon (IFN) beta-1a n = 108) [see Clinical Studies (14.2)].
In the controlled pediatric study, the safety profile in pediatric patients (10 to less than 18 years of age) receiving fingolimod 0.25 mg or 0.5 mg capsules daily was similar to that seen in adult patients. In the pediatric study, cases of seizures were reported in 5.6% of fingolimod-treated patients and 0.9% of interferon beta-1a-treated patients.
It is recommended that pediatric patients, if possible, complete all immunizations in accordance with current immunization guidelines prior to initiating TASCENSO ODT therapy.
Safety and effectiveness of TASCENSO ODT in pediatric patients below the age of 10 years have not been established.
8.5 Geriatric Use
Clinical MS studies of fingolimod capsules did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently than younger patients. TASCENSO ODT should be used with caution in patients aged 65 years and over, reflecting the greater frequency of decreased hepatic, or renal, function and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
Because fingolimod, but not fingolimod-phosphate, exposure is doubled in patients with severe hepatic impairment, patients with severe hepatic impairment should be closely monitored, as the risk of adverse reactions may be greater [see Warnings and Precautions (5.6), Clinical Pharmacology (12.3)].
No dose adjustment is needed in patients with mild or moderate hepatic impairment.
8.7 Renal Impairment
The blood level of some fingolimod metabolites is increased (up to 13-fold) in patients with severe renal impairment [see Clinical Pharmacology (12.3)]. The toxicity of these metabolites has not been fully explored. The blood level of these metabolites has not been assessed in patients with mild or moderate renal impairment.
10. Overdosage
Fingolimod, the active moiety in TASCENSO ODT, can induce bradycardia as well as AV conduction blocks (including complete AV block). The decline in heart rate usually starts within 1 hour of the first dose and is maximal within 6 hours in most patients [see Warnings and Precautions (5.1)]. In case of TASCENSO ODT overdosage, observe patients overnight with continuous ECG monitoring in a medical facility, and obtain regular measurements of blood pressure. [see Dosage and Administration (2.4)].
Neither dialysis nor plasma exchange results in removal of fingolimod from the body.
11. Tascenso ODT Description
Fingolimod is a sphingosine 1-phosphate receptor modulator.
Chemically, fingolimod lauryl sulfate is 2-amino-2-[2-(4-octylphenyl)ethyl]propan-1,3-diol lauryl sulfate. Its structure is shown below:
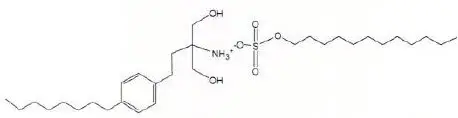
Fingolimod lauryl sulfate is a white to practically white powder that is practically insoluble in water. It has a molecular weight of 573.87 g/mol.
TASCENSO ODT is provided as 0.25 mg and 0.5 mg orally disintegrating tablets for oral use.
Each orally disintegrating tablet contains 0.25 mg or 0.5 mg of fingolimod (equivalent to 0.47 mg or 0.93 mg of fingolimod lauryl sulfate) and the following inactive ingredients: gelatin, mannitol, medium-chain triglycerides, sodium chloride, and sodium lauryl sulfate.
12. Tascenso ODT - Clinical Pharmacology
12.1 Mechanism of Action
Fingolimod is metabolized by sphingosine kinase to the active metabolite, fingolimod-phosphate. Fingolimod phosphate is a sphingosine 1-phosphate receptor modulator and binds with high affinity to sphingosine 1-phosphate receptors 1, 3, 4, and 5. Fingolimod-phosphate blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood. The mechanism by which fingolimod exerts therapeutic effects in multiple sclerosis is unknown but may involve reduction of lymphocyte migration into the central nervous system.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Oral carcinogenicity studies of fingolimod were conducted in mice and rats. In mice, fingolimod was administered at oral doses of 0, 0.025, 0.25, and 2.5 mg/kg/day for up to 2 years. The incidence of malignant lymphoma was increased in males and females at the mid and high dose. The lowest dose tested (0.025 mg/kg/day) is less than the RHD of 0.5 mg/day on a body surface area (mg/m2) basis. In rats, fingolimod was administered at oral doses of 0, 0.05, 0.15, 0.5, and 2.5 mg/kg/day. No increase in tumors was observed. The highest dose tested (2.5 mg/kg/day) is approximately 50 times the RHD on a mg/m2 basis.
Fingolimod was negative in a battery of in vitro (Ames, mouse lymphoma thymidine kinase, chromosomal aberration in mammalian cells) and in vivo (micronucleus in mouse and rat) assays.
When fingolimod was administered orally (0, 1, 3, and 10 mg/kg/day) to male and female rats prior to and during mating, and continuing to Day 7 of gestation in females, no effect on fertility was observed up to the highest dose tested (10 mg/kg), which is approximately 200 times the RHD on a mg/m2 basis.
13.2 Animal Toxicology and/or Pharmacology
Lung toxicity was observed in 2 different strains of rats and in dogs and monkeys. The primary findings included increase in lung weight, associated with smooth muscle hypertrophy, hyperdistention of the alveoli, and/or increased collagen. Insufficient or lack of pulmonary collapse at necropsy, generally correlated with microscopic changes, was observed in all species. In rats and monkeys, lung toxicity was observed at all oral doses tested in chronic studies. The lowest doses tested in rats (0.05 mg/kg/day in the 2-year carcinogenicity study) and monkeys (0.5 mg/kg/day in the 39-week toxicity study) are similar to and approximately 20 times the RHD on a mg/m2 basis, respectively.
In the 52-week oral study in monkeys, respiratory distress associated with ketamine administration was observed at doses of 3 and 10 mg/kg/day; the most affected animal became hypoxic and required oxygenation. As ketamine is not generally associated with respiratory depression, this effect was attributed to fingolimod. In a subsequent study in rats, ketamine was shown to potentiate the bronchoconstrictive effects of fingolimod. The relevance of these findings to humans is unknown.
14. Clinical Studies
The efficacy of TASCENSO ODT is based on the relative bioavailability of TASCENSO ODT orally disintegrating tablets compared to fingolimod capsules in healthy adults [see Clinical Pharmacology (12.3)].
The clinical studies described below were conducted using fingolimod capsules.
14.2 Pediatric Patients (10 to less than 18 Years of Age)
Study 4 (NCT01892722) evaluated the efficacy of once-daily oral doses of fingolimod capsules 0.25 mg or fingolimod capsules 0.5 mg in pediatric patients 10 to less than 18 years of age with relapsing-remitting multiple sclerosis. Study 4 was a 215 patient, double-blind, randomized, clinical trial that compared fingolimod to intramuscular interferon beta-1a. Prior therapy with interferon-beta, dimethyl fumarate, or glatiramer acetate up to the time of randomization was permitted. The study included patients who had experienced at least 1 clinical relapse during the year prior or 2 relapses during the 2 years prior to screening, or evidence of 1 or more Gd-enhancing lesions on MRI within 6 months prior to randomization and had an EDSS score from 0 to 5.5. Neurological evaluations were scheduled at screening, every 3 months, and at the time of suspected relapses. MRI evaluations were performed at screening and every 6 months throughout the study. The primary endpoint was the annualized relapse rate.
At baseline, the median age was 16 years, median disease duration since first symptom was 1.5 years, and median EDSS score was 1.5. One patient received no study drug and is excluded from the analysis of efficacy. Median duration of exposure to study drug was 634 days in the fingolimod group (n = 107) and 547 days in the interferon beta-1a group (n = 107). In the fingolimod group, 6.5% of patients did not complete the study, compared to 18.5% in the interferon beta-1a group.
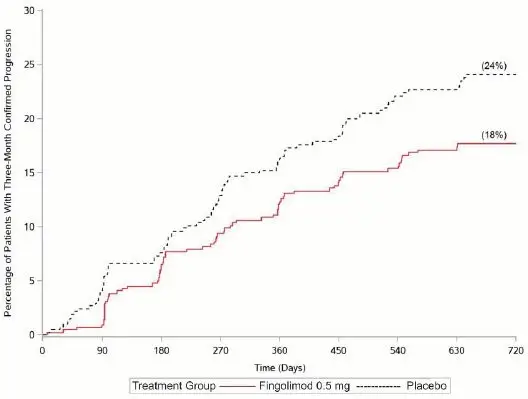
The primary endpoint, the annualized relapse rate (ARR), was significantly lower in patients treated with fingolimod (0.122) than in patients who received interferon beta-1a (0.675). Relative reduction in ARR was 81.9%. The annualized rate of the number of new or newly enlarged T2 lesions up to month 24 (key secondary endpoint) was significantly lower in patients treated with fingolimod, as was the number of Gd-enhancing T1 lesions per scan up to month 24.
Table 4 summarizes the results of Study 4.
| Fingolimod 0.25 or 0.5 mg PO N = 107 | Interferon beta-1a 30 mcg IM N = 107 | p-value | Relative Reduction |
|
|---|---|---|---|---|
| Clinical endpoints | ||||
| Annualized relapse rate (primary endpoint) | 0.122 | 0.675 | < 0.001§ | 81.9% |
| Percent of patients remaining relapse-free at 24 months | 86.0% | 45.8% | ||
| MRI endpoints | ||||
| Annualized rate of the number of new or newly enlarging T2 lesions | 4.393 | 9.269 | < 0.001§ | 52.6% |
| Mean number of Gd-enhancing T1 lesions per scan up to Month 24 | 0.436 | 0.436 | < 0.001§ | 66.0% |
All analyses of clinical endpoints were on full analysis set. MRI analyses used the evaluable dataset.
§Indicates statistical significance vs. Interferon beta-1a IM at two-sided 0.05 level.
16. How is Tascenso ODT supplied
16.1 How Supplied
0.25 mg TASCENSO ODT orally disintegrating tablets are supplied as follows:
White to off-white, round, orally disintegrating tablet debossed with 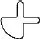 .
.
Carton of 30 orally disintegrating tablets containing 3 blister cards of 10 orally disintegrating tablets per blister card
NDC 70709-062-30
0.5 mg TASCENSO ODT orally disintegrating tablets are supplied as follows:
White to off-white, round, orally disintegrating tablet debossed with  .
.
Carton of 30 orally disintegrating tablets containing 3 blister cards of 10 orally disintegrating tablets per blister card
NDC 70709-065-30
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Tell patients not to discontinue TASCENSO ODT without first discussing this with the prescribing physician. Advise patients to contact their physician if they accidently take more TASCENSO ODT than prescribed.
| MEDICATION GUIDE TASCENSO ODT™ (tuh-SEN-soh ODT) (fingolimod) orally disintegrating tablets |
|
|---|---|
| This Medication Guide has been approved by the U.S. Food and Drug Administration | Issued: December 2022 |
| Read this Medication Guide before you start taking TASCENSO ODT and each time you get a refill. There may be new information. If you are the parent of a child who is being treated with TASCENSO ODT, the following information applies to your child. This information does not take the place of talking to your doctor about your medical condition or your treatment. | |
|
What is the most important information I should know about TASCENSO ODT?
After you take your first dose of TASCENSO ODT, and after a child takes their first dose of 0.5 mg of TASCENSO ODT when switching from the 0.25 mg dose:
Your slow heart rate will usually return to normal within 1 month after you start taking TASCENSO ODT. Call your doctor or go to the nearest hospital emergency room right away if you have any symptoms of a slow heart rate.
Human Papilloma Virus (HPV). Due to risk of HPV infection please consult your doctor for routine pap smear.
4. Progressive multifocal leukoencephalopathy (PML). PML is a rare brain infection that usually leads to death or severe disability. If PML happens, it usually happens in people with weakened immune systems but has happened in people who do not have weakened immune systems. Symptoms of PML get worse over days to weeks. Call your doctor right away if you have any new or worsening symptoms of PML, that have lasted several days, including:
|
|
|
What is TASCENSO ODT?
It is not known if TASCENSO ODT is safe and effective in children under 10 years of age. |
|
| Who should not take TASCENSO ODT? Do not take TASCENSO ODT if you:
Talk to your doctor before taking TASCENSO ODT if you have any of these conditions, or do not know if you have any of these conditions. |
|
| What should I tell my doctor before taking TASCENSO ODT? Before you take TASCENSO ODT, tell your doctor about all your medical conditions, including if you had or now have:
Tell your doctor about all the medicines you take or have recently taken, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
|
|
|
How should I take TASCENSO ODT?
|
|
| What are possible side effects of TASCENSO ODT? TASCENSO ODT may cause serious side effects, including:
These are not all of the possible side effects of TASCENSO ODT. For more information, ask your doctor or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store TASCENSO ODT?
|
|
| General information about the safe and effective use of TASCENSO ODT.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use TASCENSO ODT for a condition for which it was not prescribed. Do not give TASCENSO ODT to other people, even if they have the same symptoms that you have. It may harm them. This Medication Guide summarizes the most important information about TASCENSO ODT. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about TASCENSO ODT that is written for health professionals. |
|
|
What are the ingredients in TASCENSO ODT?
Active ingredient: fingolimod lauryl sulfate.
Inactive ingredients: gelatin, mannitol, medium-chain triglycerides, sodium chloride, and sodium lauryl sulfate. |
|
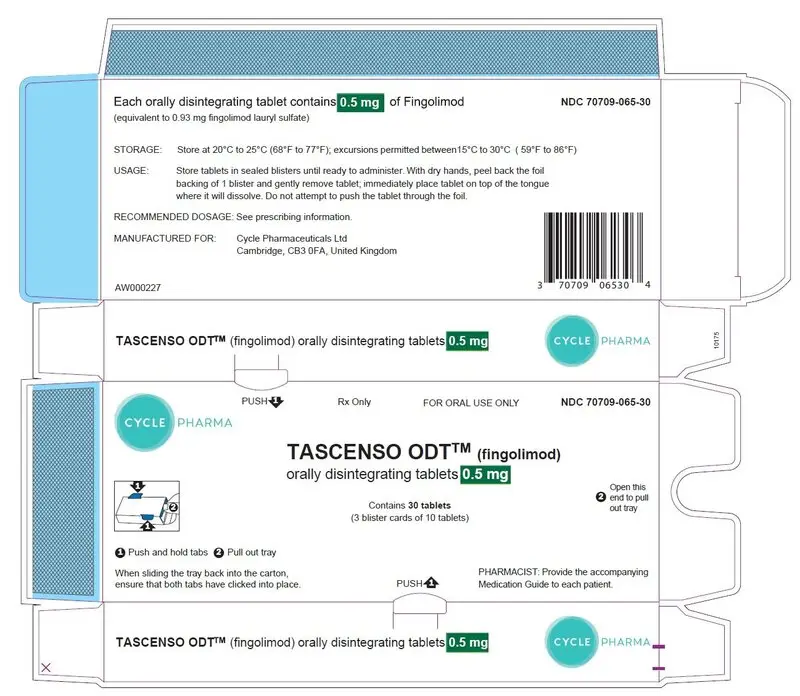
PRINCIPAL DISPLAY PANEL - 0.5 mg Tablet Carton containing Blisters
Rx Only
FOR ORAL USE ONLY
NDC 70709-065-30
CYCLE PHARMA
TASCENSO ODT™ (fingolimod)
orally disintegrating tablets 0.5 mg
Contains 30 tablets
(3 blister cards of 10 tablets)
PHARMACIST: Provide the accompanying
Medication Guide to each patient.
| TASCENSO ODT
fingolimod lauryl sulfate tablet, orally disintegrating |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| TASCENSO ODT
fingolimod lauryl sulfate tablet, orally disintegrating |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Cycle Pharmaceuticals Ltd (218215530) |
| Registrant - Cycle Pharmaceuticals Ltd (218215530) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent Pharma Solutions | 237676320 | MANUFACTURE(70709-062, 70709-065) , analysis(70709-062, 70709-065) | |