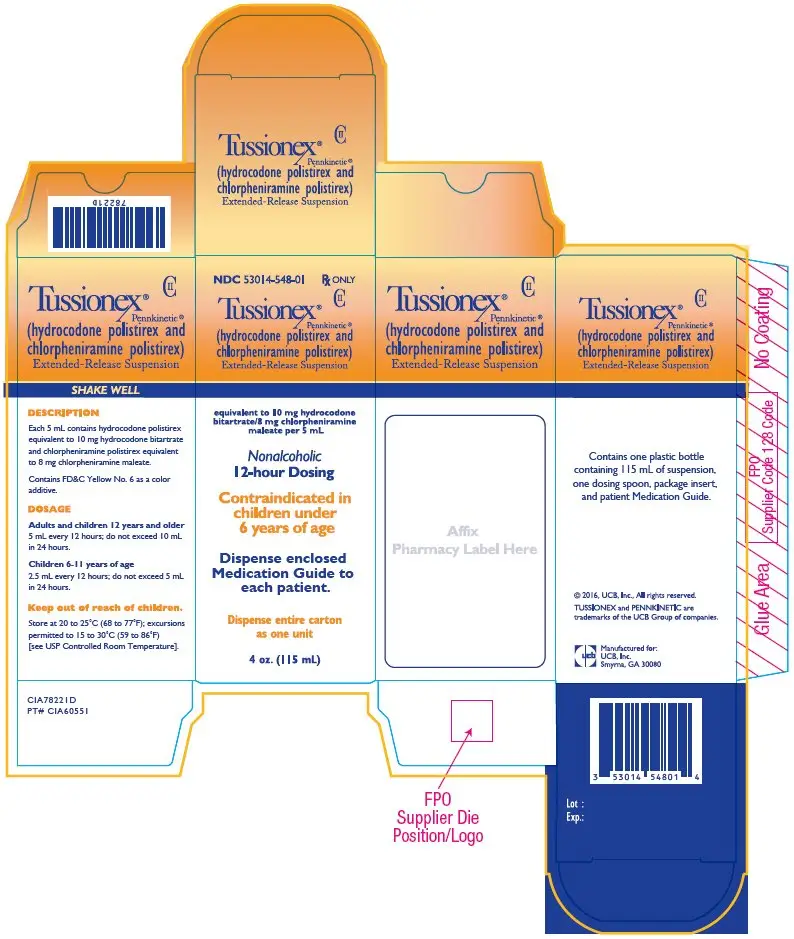
Drug Detail:Tussionex pennkinetic (Chlorpheniramine and hydrocodone> [ klor-fen-ir-a-meen-and-hye-droe-koe-done ])
Drug Class: Upper respiratory combinations
WARNING: RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
Concomitant use of opioids with benzodiazepine or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death (see WARNINGS and PRECAUTIONS – Drug Interactions). Avoid use of opioid cough medications in patients taking benzodiazepines, other CNS depressants, or alcohol.
Related/similar drugs
benzonatate, Benadryl, diphenhydramine, guaifenesin, Mucinex, dextromethorphan, chlorpheniramineTussionex - Clinical Pharmacology
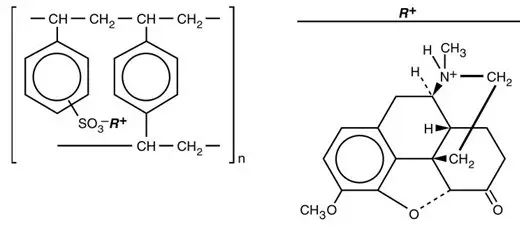
Hydrocodone is a semisynthetic narcotic antitussive and analgesic with multiple actions qualitatively similar to those of codeine. The precise mechanism of action of hydrocodone and other opiates is not known; however, hydrocodone is believed to act directly on the cough center. In excessive doses, hydrocodone, like other opium derivatives, will depress respiration. The effects of hydrocodone in therapeutic doses on the cardiovascular system are insignificant. Hydrocodone can produce miosis, euphoria, and physical and psychological dependence.
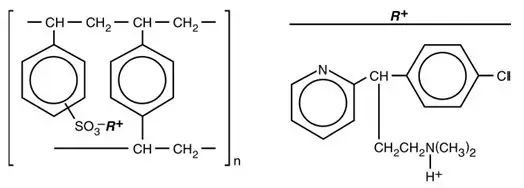
Chlorpheniramine is an antihistamine drug (H1 receptor antagonist) that also possesses anticholinergic and sedative activity. It prevents released histamine from dilating capillaries and causing edema of the respiratory mucosa.
Hydrocodone release from TUSSIONEX Pennkinetic Extended-Release Suspension is controlled by the Pennkinetic System, an extended-release drug delivery system, which combines an ion-exchange polymer matrix with a diffusion rate-limiting permeable coating. Chlorpheniramine release is prolonged by use of an ion-exchange polymer system.
Following multiple dosing with TUSSIONEX Pennkinetic Extended-Release Suspension, hydrocodone mean (S.D.) peak plasma concentrations of 22.8 (5.9) ng/mL occurred at 3.4 hours. Chlorpheniramine mean (S.D.) peak plasma concentrations of 58.4 (14.7) ng/mL occurred at 6.3 hours following multiple dosing. Peak plasma levels obtained with an immediate-release syrup occurred at approximately 1.5 hours for hydrocodone and 2.8 hours for chlorpheniramine. The plasma half-lives of hydrocodone and chlorpheniramine have been reported to be approximately 4 and 16 hours, respectively.
Contraindications
TUSSIONEX Pennkinetic Extended-Release Suspension is contraindicated in patients with a known allergy or sensitivity to hydrocodone or chlorpheniramine.
The use of TUSSIONEX Pennkinetic Extended-Release Suspension is contraindicated in children less than 6 years of age due to the risk of fatal respiratory depression.
Warnings
Pediatric Use
The use of TUSSIONEX Pennkinetic Extended-Release Suspension is contraindicated in children less than 6 years of age (see CONTRAINDICATIONS).
In pediatric patients, as well as adults, the respiratory center is sensitive to the depressant action of narcotic cough suppressants in a dose-dependent manner. Caution should be exercised when administering TUSSIONEX Pennkinetic Extended-Release Suspension to pediatric patients 6 years of age and older. Overdose or concomitant administration of TUSSIONEX Pennkinetic Extended-Release Suspension with other respiratory depressants may increase the risk of respiratory depression in pediatric patients. Benefit to risk ratio should be carefully considered, especially in pediatric patients with respiratory embarrassment (e.g., croup) (see PRECAUTIONS).
Precautions
Information for Patients
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Drug Interactions
The use of benzodiazepines, opioids, antihistamines, antipsychotics, anti-anxiety agents, or other CNS depressants (including alcohol) concomitantly with TUSSIONEX Pennkinetic Extended-Release Suspension may cause an additive CNS depressant effect, profound sedation, respiratory depression, coma, and death and should be avoided (see WARNINGS).
The use of MAO inhibitors or tricyclic antidepressants with hydrocodone preparations may increase the effect of either the antidepressant or hydrocodone.
The concurrent use of other anticholinergics with hydrocodone may produce paralytic ileus.
Adverse Reactions/Side Effects
Respiratory, Thoracic and Mediastinal Disorders
Dryness of the pharynx, occasional tightness of the chest, and respiratory depression (see CONTRAINDICATIONS).
TUSSIONEX Pennkinetic Extended-Release Suspension may produce dose-related respiratory depression by acting directly on brain stem respiratory centers (see OVERDOSAGE). Use of TUSSIONEX Pennkinetic Extended-Release Suspension in children less than 6 years of age has been associated with fatal respiratory depression. Overdose with TUSSIONEX Pennkinetic Extended-Release Suspension in children 6 years of age and older, in adolescents, and in adults has been associated with fatal respiratory depression.
| TUSSIONEX PENNKINETIC
hydrocodone polistirex and chlorpheniramine polistirex suspension, extended release |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Unither Manufacturing LLC (079176615) |