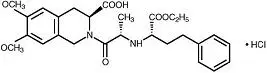
Drug Detail:Univasc (Moexipril [ moe-ex-i-pril ])
Drug Class: Angiotensin Converting Enzyme Inhibitors
Univasc - Clinical Pharmacology
Pharmacokinetics and Metabolism
Absorption
Moexipril is incompletely absorbed, with bioavailability as moexiprilat of about 13%. Bioavailability varies with formulation and food intake which reduces C max and AUC by about 70% and 40% respectively after the ingestion of a low-fat breakfast or by 80% and 50% respectively after the ingestion of a high-fat breakfast.
Warnings
Adverse Reactions/Side Effects
univasc ® has been evaluated for safety in more than 2500 patients with hypertension; more than 250 of these patients were treated for approximately one year. The overall incidence of reported adverse events was only slightly greater in patients treated with univasc ® than patients treated with placebo.
Reported adverse experiences were usually mild and transient, and there were no differences in adverse reaction rates related to gender, race, age, duration of therapy, or total daily dosage within the range of 3.75 mg to 60 mg. Discontinuation of therapy because of adverse experiences was required in 3.4% of patients treated with univasc ® and in 1.8% of patients treated with placebo. The most common reasons for discontinuation in patients treated with univasc ® were cough (0.7%) and dizziness (0.4%).
All adverse experiences considered at least possibly related to treatment that occurred at any dose in placebo-controlled trials of once-daily dosing in more than 1% of patients treated with univasc ® alone and that were at least as frequent in the univasc ® group as in the placebo group are shown in the following table:
| ADVERSE EVENT | UNIVASC
(N=674) | PLACEBO
(N=226) |
|---|---|---|
| N (%) | N (%) | |
| Cough Increased | 41 (6.1) | 5 (2.2) |
| Dizziness | 29 (4.3) | 5 (2.2) |
| Diarrhea | 21 (3.1) | 5 (2.2) |
| Flu Syndrome | 21 (3.1) | 0 (0) |
| Fatigue | 16 (2.4) | 4 (1.8) |
| Pharyngitis | 12 (1.8) | 2 (0.9) |
| Flushing | 11 (1.6) | 0 (0) |
| Rash | 11 (1.6) | 2 (0.9) |
| Myalgia | 9 (1.3) | 0 (0) |
Other adverse events occurring in more than 1% of patients on moexipril that were at least as frequent on placebo include: headache, upper respiratory infection, pain, rhinitis, dyspepsia, nausea, peripheral edema, sinusitis, chest pain, and urinary frequency. See WARNINGS and PRECAUTIONS for discussion of anaphylactoid reactions, angioedema, hypotension, neutropenia/agranulocytosis, second and third trimester fetal/neonatal morbidity and mortality, hyperkalemia, and cough.
Other potentially important adverse experiences reported in controlled or uncontrolled clinical trials in less than 1% of moexipril patients or that have been attributed to other ACE inhibitors include the following:
Cardiovascular: Symptomatic hypotension, postural hypotension, or syncope were seen in 9/1750 (0.51%) patients; these reactions led to discontinuation of therapy in controlled trials in 3/1254 (0.24%) patients who had received univasc ® monotherapy and in 1/344 (0.3%) patients who had received univasc ® with hydrochlorothiazide (see PRECAUTIONS and WARNINGS). Other adverse events included angina/myocardial infarction, palpitations, rhythm disturbances, and cerebrovascular accident.
Renal: Of hypertensive patients with no apparent preexisting renal disease, 1% of patients receiving univasc ® alone and 2% of patients receiving univasc ® with hydrochlorothiazide experienced increases in serum creatinine to at least 140% of their baseline values (see PRECAUTIONS and DOSAGE AND ADMINISTRATION).
Gastrointestinal: Abdominal pain, constipation, vomiting, appetite/weight change, dry mouth, pancreatitis, hepatitis.
Respiratory: Bronchospasm, dyspnea, eosinophilic pneumonitis.
Urogenital: Renal insufficiency, oliguria.
Dermatologic: Apparent hypersensitivity reactions manifested by urticaria, rash, pemphigus, pruritus, photosensitivity, alopecia.
Neurological and Psychiatric: Drowsiness, sleep disturbances, nervousness, mood changes, anxiety.
Other: Angioedema (see WARNINGS), taste disturbances, tinnitus, sweating, malaise, arthralgia, hemolytic anemia.
| UNIVASC
moexipril hydrochloride tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| UNIVASC
moexipril hydrochloride tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - UCB, Inc. (028526403) |