Drug Detail:Vistogard (Uridine triacetate [ ure-i-deen-trye-as-e-tate ])
Drug Class: Antidotes Miscellaneous metabolic agents
Highlights of Prescribing Information
VISTOGARD® (uridine triacetate) oral granules
Initial U.S. Approval: 2015
Indications and Usage for Vistogard
VISTOGARD® is a pyrimidine analog indicated for the emergency treatment of adult and pediatric patients:
- following a fluorouracil or capecitabine overdose regardless of the presence of symptoms, or
- who exhibit early-onset, severe or life-threatening toxicity affecting the cardiac or central nervous system, and/or early-onset, unusually severe adverse reactions (e.g., gastrointestinal toxicity and/or neutropenia) within 96 hours following the end of fluorouracil or capecitabine administration. (1)
Limitations of use:
- VISTOGARD is not recommended for the non-emergent treatment of adverse reactions associated with fluorouracil or capecitabine because it may diminish the efficacy of these drugs. (1)
- The safety and efficacy of VISTOGARD initiated more than 96 hours following the end of fluorouracil or capecitabine administration have not been established. (1)
Vistogard Dosage and Administration
Recommended Dosage
- Adults: 10 grams (1 packet) orally every 6 hours for 20 doses, without regard to meals. (2.1)
- Pediatric: 6.2 grams/m2 of body surface area (not to exceed 10 grams per dose) orally every 6 hours for 20 doses, without regard to meals. See the full prescribing information for body surface area-based dosing. (2.1)
Preparation and Administration
- Pediatric: Measure the dose using either a scale accurate to at least 0.1 gram, or a graduated teaspoon accurate to ¼ teaspoon. (2.1)
- Mix each VISTOGARD dose with 3 to 4 ounces of soft foods such as applesauce, pudding or yogurt and ingest within 30 minutes of mixing. Do not chew the VISTOGARD granules. Drink at least 4 ounces of water. (2.2)
- If a patient vomits within 2 hours of taking a dose of VISTOGARD, initiate another complete dose as soon as possible after the vomiting episode. Administer the next dose at the regularly scheduled time. (2.2)
- If a patient misses a dose at the scheduled time, administer that dose of VISTOGARD as soon as possible. Administer the next dose at the regularly scheduled time. (2.2)
- Administer VISTOGARD via a nasogastric tube (NG tube) or gastrostomy tube (G-Tube) when necessary (e.g., severe mucositis or coma). (2.2)
Dosage Forms and Strengths
Oral granules: 10 gram packets (3)
Contraindications
None. (4)
Warnings and Precautions
None. (5)
Adverse Reactions/Side Effects
Adverse reactions occurring in >2% of patients receiving VISTOGARD included vomiting, nausea, and diarrhea. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Wellstat Therapeutics Corporation at (1-800-914-0071) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2017
Related/similar drugs
uridineFull Prescribing Information
1. Indications and Usage for Vistogard
VISTOGARD® is indicated for the emergency treatment of adult and pediatric patients:
- following a fluorouracil or capecitabine overdose regardless of the presence of symptoms, or
- who exhibit early-onset, severe or life-threatening toxicity affecting the cardiac or central nervous system, and/or early-onset, unusually severe adverse reactions (e.g., gastrointestinal toxicity and/or neutropenia) within 96 hours following the end of fluorouracil or capecitabine administration.
2. Vistogard Dosage and Administration
2.1 Recommended Dosage
- Adults: 10 grams (1 packet) orally every 6 hours for 20 doses, without regard to meals.
Pediatric: 6.2 grams/m2 of body surface area (not to exceed 10 grams per dose) orally every 6 hours for 20 doses, without regard to meals. The VISTOGARD dose to be administered at 6.2 grams/m2 is presented in Table 1. Measure the dose using either a scale accurate to at least 0.1 gram, or a graduated teaspoon accurate to ¼ teaspoon. Discard any unused portion of granules. Do not use granules left in the open packet for subsequent dosing.Table 1 VISTOGARD Pediatric Dose Based on Body Surface Area (m2) Patient Body Surface Area
(m2)Table 1 VISTOGARD 6.2 grams/m2/dose* Dose in Grams Dose in Graduated Teaspoons - *
- Dose by body surface area category in this table was rounded to achieve the approximate dose. Each dose is administered every 6 hours for 20 doses.
- †
- May use 1 entire 10 g packet without weighing or measuring. Do not exceed 10 grams/dose.
0.34 to 0.44 2.1 to 2.7 1 0.45 to 0.55 2.8 to 3.4 1 ¼ 0.56 to 0.66 3.5 to 4.1 1 ½ 0.67 to 0.77 4.2 to 4.8 1 ¾ 0.78 to 0.88 4.9 to 5.4 2 0.89 to 0.99 5.5 to 6.1 2 ¼ 1.00 to 1.10 6.2 to 6.8 2 ½ 1.11 to 1.21 6.9 to 7.5 2 ¾ 1.22 to 1.32 7.6 to 8.1 3 1.33 to 1.43 8.2 to 8.8 3 ¼ 1.44 and above † 10.0 1 full packet † - Administer VISTOGARD as soon as possible after an overdose or early-onset toxicity within 96 hours following the end of fluorouracil or capecitabine administration.
- Administer full course of VISTOGARD (20 doses) as directed.
2.2 Preparation and Administration
- Mix each VISTOGARD dose with 3 to 4 ounces of soft foods such as applesauce, pudding or yogurt and ingest within 30 minutes. Do not chew the VISTOGARD granules. Drink at least 4 ounces of water.
- If a patient vomits within 2 hours of taking a dose of VISTOGARD, initiate another complete dose as soon as possible after the vomiting episode. Administer the next dose at the regularly scheduled time.
- If a patient misses a dose at the scheduled time, administer that dose of VISTOGARD as soon as possible. Administer the next dose at the regularly scheduled time.
- Administer VISTOGARD via a nasogastric tube (NG tube) or gastrostomy tube (G-Tube) when necessary (eg, severe mucositis or coma). Follow the instructions below for each dose administration:
- Prepare approximately 4 fluid ounces (about 100 mL) of a food starch-based thickening product in water and stir briskly until the thickener has dissolved.
- Crush the contents of one full 10 gram packet of VISTOGARD granules to a fine powder.
- Add the crushed VISTOGARD granules to 4 ounces (about 100 mL) of the reconstituted food starch-based thickening product. For pediatric patients receiving less than 10 grams, prepare the mixture at a ratio of no greater than 1 gram per 10 mL of reconstituted food starch-based thickening product and mix thoroughly.
- After administration of the mixture using the NG tube or G-Tube, flush the tube with water.
3. Dosage Forms and Strengths
Oral granules: 10 grams of orange-flavored, white-to-off-white, oral granules (95% w/w) in single-dose packets.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions and using a wide range of doses, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of VISTOGARD was assessed in 135 patients (median age 59 years, 56% male) treated in 2 single-arm, open-label, multi-center trials. VISTOGARD was administered at 10 grams orally every 6 hours for 20 doses or at a body surface area adjusted dosage of 6.2 grams/m2/dose for 20 doses in four patients between 1 and 7 years of age. The median duration of exposure was 4.8 days, with a median of 20 doses (range 1 to 23). VISTOGARD was discontinued for adverse reactions in two (1.4%) patients. Serious adverse reactions and Grade ≥3 adverse reactions were seen in one patient receiving VISTOGARD (Grade 3 nausea and vomiting). Table 2 summarizes the adverse reactions that occurred in greater than 2% of patients in Studies 1 and 2 combined.
| Adverse Reaction | N=135 Patients |
|---|---|
| Vomiting | 13 (10%) |
| Nausea | 7 (5%) |
| Diarrhea | 4 (3%) |
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of VISTOGARD have been established in pediatric patients. Use of VISTOGARD in pediatric patients is supported by an open-label clinical study of adults (Study 1) and a second open-label clinical study which included 6 pediatric patients ranging in age from 1 to 16 years (Study 2). Four of these pediatric patients were between 1 to 7 years of age and received a body-surface area adjusted dosage of 6.2 grams/m2/dose for 20 doses. The clinical response and safety in adult and pediatric patients treated with VISTOGARD were similar; however, clinical data are limited [see Clinical Studies (14)].
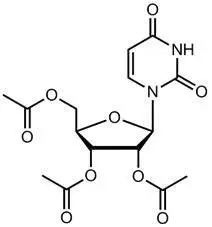
11. Vistogard Description
VISTOGARD oral granules contain the active ingredient uridine triacetate which is a pyrimidine analog. The chemical name for uridine triacetate is (2',3',5'-tri-O-acetyl-β-D-ribofuranosyl)-2,4(1H,3H)-pyrimidinedione. The molecular weight is 370.3 grams/mole and it has an empirical formula of C15H18N2O9. The structural formula is:
Each single-dose 10 gram packet of VISTOGARD orange-flavored oral granules (95% w/w) contains 10 grams of uridine triacetate and the following inactive ingredients: ethylcellulose (0.309 grams), Opadry® Clear [proprietary dispersion of hydroxypropylmethylcellulose and Macrogol] (0.077 grams), and natural orange juice flavor (0.131 grams).
12. Vistogard - Clinical Pharmacology
12.1 Mechanism of Action
Uridine triacetate is an acetylated pro-drug of uridine. Following oral administration, uridine triacetate is deacetylated by nonspecific esterases present throughout the body, yielding uridine in the circulation. Uridine competitively inhibits cell damage and cell death caused by fluorouracil.
Fluorouracil is a cytotoxic antimetabolite that interferes with nucleic acid metabolism in normal and cancer cells. Cells anabolize fluorouracil to the cytotoxic intermediates 5-fluoro-2'-deoxyuridine-5'-monophosphate (FdUMP) and 5-fluorouridine triphosphate (FUTP). FdUMP inhibits thymidylate synthase, blocking thymidine synthesis. Thymidine is required for DNA replication and repair. Uridine is not found in DNA.
The second source of fluorouracil cytotoxicity is the incorporation of its metabolite, FUTP, into RNA. This incorporation of FUTP into RNA is proportional to systemic fluorouracil exposure. Excess circulating uridine derived from VISTOGARD is converted into uridine triphosphate (UTP), which competes with FUTP for incorporation into RNA.
12.3 Pharmacokinetics
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of uridine triacetate.
Uridine triacetate was not genotoxic in the Ames test, the mouse lymphoma assay and the mouse micronucleus test.
Orally administered uridine triacetate did not affect fertility or general reproductive performance in male and female rats at doses up to 2000 mg/kg per day (about one-half the maximum recommend human dose (MRHD) of 40 grams per day on a body surface area basis).
13.2 Animal Toxicology and/or Pharmacology
In mice given a sub-lethal dose of fluorouracil, the administration of oral uridine triacetate diminished hematological toxicity as a function of increasing dose, but did not completely prevent hematological toxicity. In mice given a lethal dose of fluorouracil, administration of oral uridine triacetate increased survival to 90% when given within 24 hours. Survival diminished with increasing interval between the fluorouracil dose and uridine triacetate treatment demonstrating that earlier administration of uridine triacetate is more beneficial. In similar experiments in mice, uridine triacetate treatment diminished damage to the intestinal mucosa caused by fluorouracil treatment.
14. Clinical Studies
The efficacy of VISTOGARD was assessed in 135 patients who were treated in two open-label trials, Study 1 (n=60) and Study 2 (n=75). The patients in both studies had either received an overdose of fluorouracil or capecitabine, or presented with severe or life-threatening toxicities within 96 hours following the end of fluorouracil or capecitabine administration. Overdose was defined as administration of fluorouracil at a dose, or infusion rate, greater than the intended dose or maximum tolerated dose for the patient's intended regimen of fluorouracil. VISTOGARD was administered at 10 grams orally every 6 hours for 20 doses or at a body surface area adjusted dosage of 6.2 grams/m2/dose for 20 doses for four patients between 1 and 7 years of age. The major efficacy outcome was survival at 30 days or until the resumption of chemotherapy if prior to 30 days.
In Study 1 and Study 2 combined, the median age of the patients was 59 years (range: 1 to 83), 56% were male, 72% were white, 9% were Black/African American, 6% were Hispanic, 4% were Asian, and 95% had a cancer diagnosis. Of the 135 patients, 117 were treated with VISTOGARD following an overdose of fluorouracil (n=112) or capecitabine (n=5), and 18 were treated after exhibiting severe or life-threatening fluorouracil toxicities within 96 hours following the end of fluorouracil administration. The severe or life-threatening toxicities involved the central nervous system (such as encephalopathy, acute mental status change), cardiovascular system, gastrointestinal system (such as mucositis), and bone marrow. A total of six pediatric patients were administered VISTOGARD. Four patients initiated VISTOGARD more than 96 hours following the end of fluorouracil or capecitabine administration. Of the 112 patients overdosed with fluorouracil, 105 (94%) were overdosed by infusion rate only (range 1.3 to 720 times the planned infusion rate), four (4%) were overdosed by dose only, and three (3%) were overdosed by both dose and rate.
The survival results are shown in Table 4. Of the 135 patients who were treated with VISTOGARD in Studies 1 and 2 there were five deaths due to fluorouracil or capecitabine toxicity (4%). Of the five patients who died, two were treated after 96 hours following the end of fluorouracil administration. In the patients treated with VISTOGARD for an overdose of fluorouracil or capecitabine in Studies 1 and 2 combined (n=117), survival at 30 days was 97% (n=114). In the patients receiving VISTOGARD for early-onset severe or life-threatening toxicity in Studies 1 and 2 combined (n=18), the survival at 30 days was 89% (n=16). In these studies 33% of patients (n=45) resumed chemotherapy in less than 30 days. Based on retrospective historical case reports of 25 patients who were overdosed with fluorouracil and received supportive care only, all were overdosed by rate with a range 1.9 to 64 times the planned infusion rate, and 84% died.
| Overdose | Early-Onset | Overall | |
|---|---|---|---|
|
|||
| Total Enrolled | 117 | 18 | 135 |
| Survival* | 114 (97%) | 16 (89%) | 130 (96%) |
| Death | 3 (3%) | 2 (11%) | 5 (4%) |
16. How is Vistogard supplied
VISTOGARD orange-flavored oral granules (95% w/w) are available in single-dose packets containing 10 grams of uridine triacetate.
| NDC Number | Package Configuration |
|---|---|
| 69468-151-20 | Course of therapy package: |
| 1 carton containing 20 single-dose packets of VISTOGARD | |
| 69468-151-04 | 24-Hour Pack: |
| 1 carton containing 4 single-dose packets of VISTOGARD |
| VISTOGARD
uridine triacetate granule |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - Wellstat Therapeutics Corporation (785233024) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Almac Pharma Services | 233170864 | MANUFACTURE(69468-151) , ANALYSIS(69468-151) , PACK(69468-151) , LABEL(69468-151) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Almac Sciences | 232665666 | API MANUFACTURE(69468-151) , ANALYSIS(69468-151) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Butterworth Laboratories Limited | 225081538 | ANALYSIS(69468-151) | |