Drug Detail:Zimhi (Naloxone hydrochloride)
Drug Class: Antidotes
Highlights of Prescribing Information
ZIMHI™ (naloxone hydrochloride injection) for intramuscular or subcutaneous use
Initial U.S. Approval: 1971
Indications and Usage for Zimhi
ZIMHI is an opioid antagonist indicated in adult and pediatric patients for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression. (1)
ZIMHI is intended for immediate administration as emergency therapy in settings where opioids may be present. (1)
ZIMHI is not a substitute for emergency medical care. (1)
Zimhi Dosage and Administration
- ZIMHI is for intramuscular or subcutaneous use only. (2.1)
- Seek emergency medical care immediately after use. (2.1)
- ZIMHI is intended to be administered by individuals 12 years of age or older. (2.1)
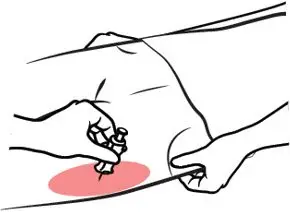
- Administer ZIMHI to adult or pediatric patients into the anterolateral aspect of the thigh, through clothing if necessary. (2.1)
- See the Full Prescribing Information and Instructions for Use for important information on how to safely administer ZIMHI. (2.1)
- Keep the patient under continued surveillance until emergency personnel arrive and administer repeated doses of ZIMHI as necessary. (2.1)
- Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance. (2.2)
- In pediatric patients under the age of one, the caregiver should pinch the thigh muscle while administering the dose. (2.2)
Dosage Forms and Strengths
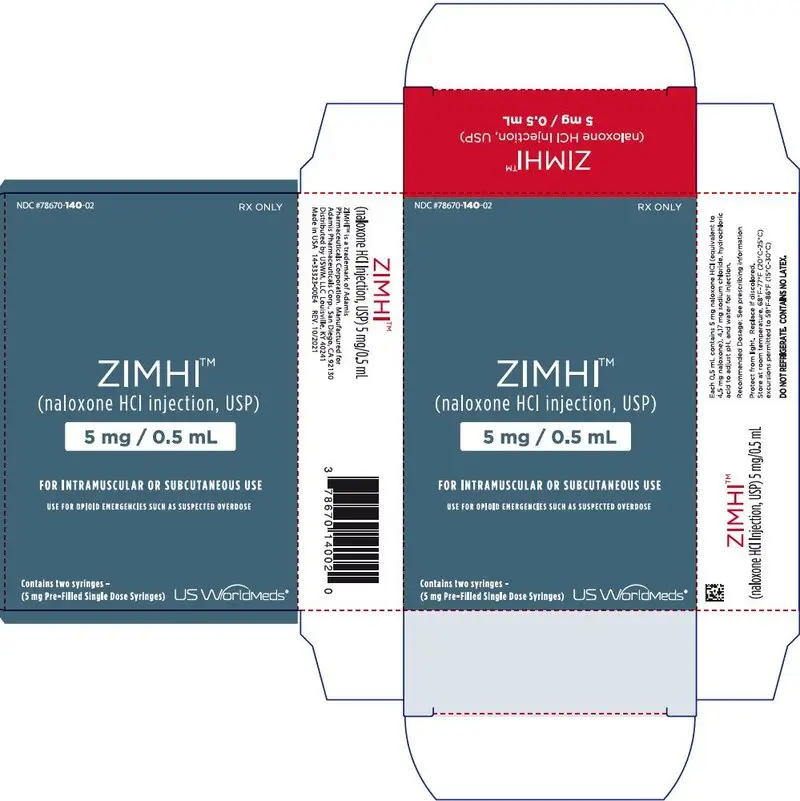
Injection: 5 mg/0.5 mL naloxone hydrochloride solution in a single-dose, pre-filled syringe. (3)
Contraindications
Hypersensitivity to naloxone hydrochloride or to any of the other ingredients in ZIMHI. (4)
Warnings and Precautions
- Risk of Recurrent Respiratory and CNS Depression: Due to the duration of action of naloxone relative to the opioid, respiratory and CNS depression may recur after the first dose of naloxone. Seek emergency medical assistance immediately after the first dose, keep the patient under continued surveillance, and administer repeated doses of naloxone using a new ZIMHI, as necessary, while awaiting emergency medical assistance. (5.1)
- Risk of Limited Efficacy with Partial Agonists or Mixed Agonists/Antagonists: Reversal of respiratory depression caused by partial agonists or mixed agonists/antagonists such as buprenorphine and pentazocine, may be incomplete. Larger or repeat doses may be required. (5.2)
- Precipitation of Severe Opioid Withdrawal: Use in patients who are opioid dependent results in rapid onset of opioid withdrawal. In neonates, opioid withdrawal may be life-threatening if not recognized and properly treated. Monitor for the development of opioid withdrawal. (5.3)
- Risk of Cardiovascular (CV) Effects: Abrupt postoperative reversal of opioid depression may result in adverse CV effects. These events have primarily occurred in patients who had pre-existing CV disorders or received other drugs that may have similar CV effects. Monitor these patients closely in an appropriate healthcare setting after use of naloxone hydrochloride. (5.3)
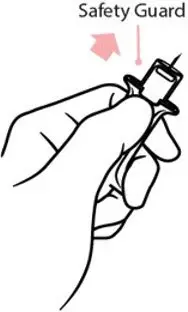
- Risk of Accidental Needlestick Injury: After use, the ZIMHI needle is exposed until the safety guard is deployed. Stress to patients the importance of familiarizing themselves with the device and its operation prior to experiencing an emergency situation, and to immediately seek medical attention should a needlestick occur. (5.4)
Adverse Reactions/Side Effects
The following adverse reactions were most commonly observed in ZIMHI clinical studies: nausea, dizziness, lightheadedness, and elevated bilirubin. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Adamis Pharmaceuticals Corporation at 1-800-230-3935 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2021
Related/similar drugs
naloxone, Narcan, Opvee, Evzio, nalmefeneFull Prescribing Information
1. Indications and Usage for Zimhi
ZIMHI™ is indicated in adults and pediatric patients for:
- the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression.
ZIMHI is intended for immediate administration as emergency therapy in settings where opioids may be present.
ZIMHI is not a substitute for emergency medical care.
2. Zimhi Dosage and Administration
2.1 Important Administration Instructions
ZIMHI is for intramuscular and subcutaneous use only.
Because treatment of known or suspected opioid overdose must be performed by someone other than the patient, instruct the prescription recipient to inform those around them about the presence of ZIMHI and the Instructions for Use.
ZIMHI is intended to be administered by individuals 12 years of age or older. Younger individuals or those with limited hand strength may find the device difficult to use.
ZIMHI is light sensitive. Store ZIMHI in the outer case provided to protect it from light.
Prior to a medical emergency (during storage), periodically visually inspect ZIMHI™ through the viewing window on the device. If the solution is discolored yellow or brown color, cloudy, or contains particles, replace ZIMHI™ with a new one [see How Supplied/Storage and Handling (16.2)].
Do not attempt to reuse ZIMHI. Each ZIMHI contains a single dose of naloxone hydrochloride for single-dose injection.
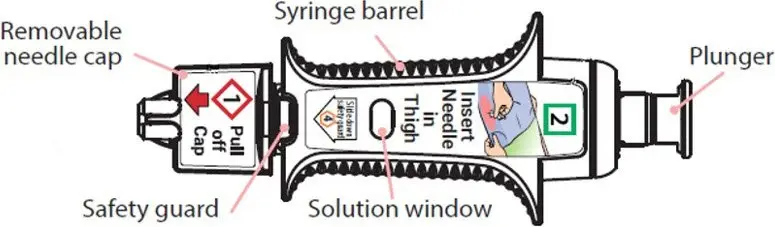
Instruct the patient or caregiver to read the Instructions for Use at the time they receive a prescription for ZIMHI™. Emphasize the following instructions to the patient or caregiver:
- Seek emergency medical care immediately after use. Because the duration of action of most opioids exceeds that of naloxone hydrochloride and the suspected opioid overdose may occur outside of supervised medical settings, seek immediate emergency medical assistance, keep the patient under continued surveillance until emergency personnel arrive, and administer repeated doses of ZIMHI as necessary. Always seek emergency medical assistance in the event of a suspected potentially life-threatening opioid emergency after administration of the first dose of ZIMHI.
- Additional doses of ZIMHI may be required until emergency medical assistance becomes available [see Dosage and Administration (2.2)].
- Administer ZIMHI according to the Instructions for Use and the printed instructions on the device label:
- –
- Place the patient in the supine position.
- –
- Inject ZIMHI intramuscularly or subcutaneously into the anterolateral aspect of the thigh with the needle facing downwards. Inject through clothing if necessary [see Instructions for Use].
- –
- Embed the needle completely before transferring the thumb to the syringe plunger.
- –
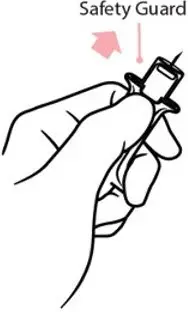
- Immediately after injection, using one hand with fingers behind the needle, slide the safety guard over the needle. Do not use two hands to activate the safety guard.
- –
- Never put thumb, fingers, or hand over the exposed needle. Failure to follow these instructions may result in a needlestick injury. If an accidental needlestick occurs, get medical help immediately [see Warnings and Precautions (5.4)].
- –
- Do NOT attempt to re-cap the needle with the needle cap once it has been removed [see Warnings and Precautions (5.4)].
- –
- Place the patient in the lateral recumbent position (recovery position).
- –
- ZIMHI must be used and/or properly disposed of as described below once the protective cap covering the needle is removed.
- –
- Put the used syringe into the blue case, close the case, and give your used ZIMHI syringe to the healthcare provider for inspection and proper disposal.
- –
- Tell the healthcare provider that you have received or administered an injection of naloxone HCl. Show the healthcare provider where the injection was administered.
3. Dosage Forms and Strengths
Injection: 5 mg/0.5 mL naloxone hydrochloride is a clear, colorless to slightly yellow, sterile solution in a single-dose, pre-filled syringe. Each ZIMHI 5 mg delivers 5 mg naloxone hydrochloride (equivalent to 4.5 mg naloxone) injection, USP (0.5 mL).
4. Contraindications
ZIMHI is contraindicated in patients known to be hypersensitive to naloxone hydrochloride or to any of the other ingredients.
5. Warnings and Precautions
5.1 Risk of Recurrent Respiratory and Central Nervous System Depression
The duration of action of most opioids may exceed that of ZIMHI resulting in a return of respiratory and/or central nervous system depression after an initial improvement in symptoms. Therefore, it is necessary to seek emergency medical assistance immediately after delivering the first dose of ZIMHI. Keep the patient under continued surveillance and administer additional doses of ZIMHI if the patient is not adequately responding or responds and then relapses back into respiratory depression, as necessary [see Dosage and Administration (2.2)]. Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance.
5.2 Risk of Limited Efficacy with Partial Agonists or Mixed Agonist/Antagonists
Reversal of respiratory depression by partial agonists or mixed agonist/antagonists such as buprenorphine and pentazocine, may be incomplete. Larger or repeat doses of naloxone hydrochloride may be required to antagonize buprenorphine because the latter has a long duration of action due to its slow rate of binding and subsequent slow dissociation from the opioid receptor [see Dosage and Administration (2)]. Buprenorphine antagonism is characterized by a gradual onset of the reversal effects and a decreased duration of action of the normally prolonged respiratory depression.
5.3 Precipitation of Severe Opioid Withdrawal
The use of ZIMHI in patients who are opioid dependent results in opioid withdrawal characterized by rapid onset of severe body aches, vomiting, diarrhea, tachycardia, fever, runny nose, sneezing, piloerection, sweating, yawning, nausea, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure. In neonates, opioid withdrawal may be life-threatening if not recognized and properly treated and may include the following signs and symptoms: convulsions, excessive crying, and hyperactive reflexes. Monitor patients for the development of the signs and symptoms of opioid withdrawal.
Abrupt postoperative reversal of opioid depression after using naloxone hydrochloride may result in nausea, vomiting, sweating, tremulousness, tachycardia, hypotension, hypertension, seizures, ventricular tachycardia and fibrillation, pulmonary edema, and cardiac arrest. Death, coma, and encephalopathy have been reported as sequelae of these events. These events have primarily occurred in patients who had pre-existing cardiovascular disorders or received other drugs that may have similar adverse cardiovascular effects. Although a direct cause and effect relationship has not been established, after use of naloxone hydrochloride, monitor patients with pre-existing cardiac disease or patients who have received medications with potential adverse cardiovascular effects for hypotension, ventricular tachycardia or fibrillation, and pulmonary edema in an appropriate healthcare setting. It has been suggested that the pathogenesis of pulmonary edema associated with the use of naloxone hydrochloride is similar to neurogenic pulmonary edema, i.e., a centrally mediated massive catecholamine response leading to a dramatic shift of blood volume into the pulmonary vascular bed resulting in increased hydrostatic pressures.
There may be clinical settings, particularly the postpartum period in neonates with known or suspected exposure to maternal opioid use, where it is preferable to avoid the abrupt precipitation of opioid withdrawal symptoms. In these settings, consider use of an alternative naloxone product which can be titrated to effect and, where applicable, dosed according to weight [see Use in Specific Populations (8.4)].
5.4 Risk of Accidental Needlestick Injury
After use, the ZIMHI needle is exposed until the safety guard is deployed. A needlestick injury could occur during use in emergency situations. In the event that an accidental needlestick occurs, medical attention should be sought. Potential exposure to blood borne pathogens including HIV, HBV, and HCV requires immediate evaluation by a medical professional. Stress to patients the importance of familiarizing themselves with the device and its operation prior to experiencing an emergency situation, so they are familiar with the safety guard and its deployment.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed elsewhere in the labeling:
- Precipitation of Severe Opioid Withdrawal [see Warnings and Precautions (5.3)]
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to the rates in the clinical studies of another drug and may not reflect the rates observed in practice.
The following adverse reactions were observed in ZIMHI™ clinical studies in healthy volunteers without opioid dependence: nausea, dizziness, lightheadedness, and elevated bilirubin.
The following adverse reactions have been identified during post-approval use of naloxone hydrochloride in the post-operative setting. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: Hypotension, hypertension, ventricular tachycardia and fibrillation, dyspnea, pulmonary edema, and cardiac arrest. Death, coma, and encephalopathy have been reported as sequelae of these events. Excessive doses of naloxone hydrochloride in post-operative patients have resulted in significant reversal of analgesia and have caused agitation [see Warnings and Precautions (5.3)].
Other events that have been reported in post-marketing use of naloxone hydrochloride include agitation, disorientation, confusion, and anger.
Abrupt reversal of opioid effects in persons who were opioid dependent resulted in opioid withdrawal characterized by rapid onset of severe body aches, vomiting, diarrhea, tachycardia, fever, runny nose, sneezing, piloerection, sweating, yawning, nausea, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness and increased blood pressure. In some patients, aggressive behavior has occurred in the context of abrupt reversal of an opioid overdose. In the neonate, opioid withdrawal signs and symptoms also included: convulsions, excessive crying, hyperactive reflexes [see Warnings and Precautions (5.3)].
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of ZIMHI™ (for intramuscular and subcutaneous use) have been established in pediatric patients of all ages for the emergency treatment of known or suspected opioid overdose as manifested by respiratory and/or central nervous system depression. Use of naloxone hydrochloride in all pediatric patients is supported by adult bioequivalence studies coupled with evidence from the safe and effective use of another naloxone hydrochloride injectable product. No pediatric studies were conducted for ZIMHI.
Absorption of naloxone hydrochloride following subcutaneous or intramuscular administration in pediatric patients may be erratic or delayed. Even when the opiate-intoxicated pediatric patient responds appropriately to naloxone hydrochloride injection, he/she must be carefully monitored for at least 24 hours as a relapse may occur as naloxone is metabolized.
In opioid-dependent pediatric patients, (including neonates), administration of naloxone hydrochloride may result in an abrupt and complete reversal of opioid effects, precipitating an acute opioid withdrawal syndrome. There may be clinical settings, particularly the postpartum period in neonates with known or suspected exposure to maternal opioid use, where it is preferable to avoid the abrupt precipitation of opioid withdrawal symptoms. Unlike acute opioid withdrawal in adults, acute opioid withdrawal in neonates manifesting as seizures may be life-threatening if not recognized and properly treated. Other signs and symptoms in neonates may include excessive crying and hyperactive reflexes. In these settings where it may be preferable to avoid the abrupt precipitation of acute opioid withdrawal symptoms, consider use of an alternative, naloxone hydrochloride product that can be dosed according to weight and titrated to effect. [see Warnings and Precautions (5.3)].
In pediatric patients under the age of one year, the caregiver should pinch the thigh muscle while administering ZIMHI. Carefully observe the administration site for evidence of residual needle parts, signs of infection, or both. [see Dosing Information (2.2)].
8.5 Geriatric Use
Geriatric patients have a greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy. Therefore, the systemic exposure of naloxone can be higher in these patients.
Clinical studies of naloxone hydrochloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
11. Zimhi Description
ZIMHI (naloxone hydrochloride injection, USP) is an opioid antagonist supplied in a single-dose pre-filled syringe. ZIMHI is not made with natural rubber latex. Chemically, naloxone hydrochloride is the hydrochloride salt of 17-Allyl-4,5α-epoxy-3,14- dihydroxymorphinan-6-one hydrochloride with the following structure:
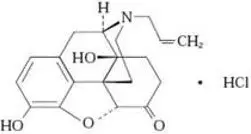 |
| C19H21NO4∙ HCl |
| 363.8 g/mol |
Naloxone hydrochloride occurs as a white to slightly off-white powder, and is soluble in water, in dilute acids, and in strong alkali; slightly soluble in alcohol; practically insoluble in ether and in chloroform.
Each 0.5 mL contains 5 mg naloxone hydrochloride (equivalent to 4.5 mg naloxone), 4.17 mg sodium chloride, hydrochloric acid to adjust pH, and water for injection.
12. Zimhi - Clinical Pharmacology
12.1 Mechanism of Action
Naloxone hydrochloride is an opioid antagonist that antagonizes opioid effects by competing for the same receptor sites.
Naloxone hydrochloride reverses the effects of opioids, including respiratory depression, sedation, and hypotension. Also, it can reverse the psychotomimetic and dysphoric effects of agonist-antagonists such as pentazocine.
12.2 Pharmacodynamics
When naloxone hydrochloride is administered intravenously, the onset of action is generally apparent within two minutes. The time to onset of action is shorter for intravenous compared to subcutaneous or intramuscular routes of administration.
The duration of action is dependent upon the dose and route of administration of naloxone hydrochloride.
12.3 Pharmacokinetics
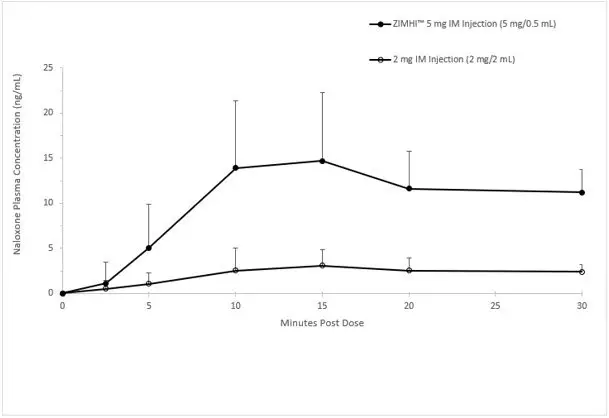
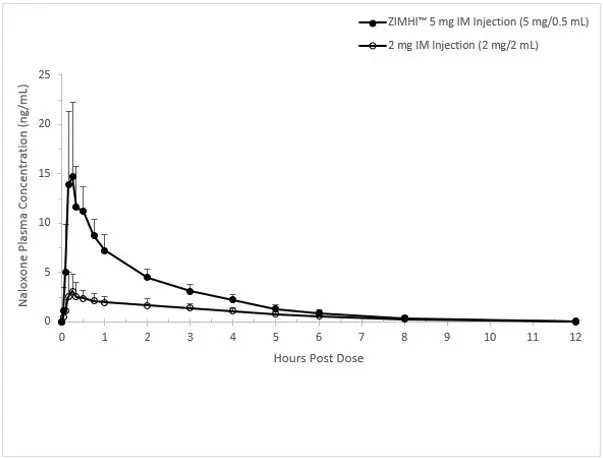
In a pharmacokinetic study of 14 healthy adults, a single intramuscular injection of 5 mg ZIMHI in a single-dose pre-filled syringe in a delivery device provides significantly higher Cmax and AUC compared to a single intramuscular injection of 2 mg naloxone hydrochloride (1mg /1mL). Naloxone plasma concentration versus time profiles are shown in Figure 1.
The pharmacokinetic parameters of naloxone in plasma are shown in Table 1.
| Parameter | Intramuscular Injection of 5 mg ZIMHI (N = 14) | Intramuscular Injection of 2 mg Naloxone HCl (N = 14) |
|---|---|---|
|
||
| Tmax (h)* | 0.25 (0.17, 0.52) | 0.25 (0.05, 3.00) |
| Cmax (ng/mL) | 17.2 (44) | 3.58 (58.1) |
| AUC0-t (ng.h/mL) | 26.2 (21.5) | 9.43 (23.8) |
| AUC0-inf (ng.h/mL) | 26.6 (21.2) | 9.97 (22.6) |
| AUC0-0.04h (ng.h/mL) | 0.02 (208) | 0.01 (164) |
| AUC0-0.08h (ng.h/mL) | 0.15 (117) | 0.04 (128) |
| T1/2 (h) | 1.50 (15.2) | 1.86 (28.9) |
Figure 1 Mean ± SD Plasma Concentration of Naloxone, (a) 0-30 min and (b) 0-12 h Following a Single Intramuscular Administration of 5 mg ZIMHI and a Single Intramuscular Administration of 2 mg Naloxone HCl
a)
b)
16. How is Zimhi supplied
16.1 How Supplied
ZIMHI (naloxone hydrochloride injection, USP) 5 mg/0.5 mL is a clear, colorless to slightly yellow, sterile solution provided as follows:
- NDC78670-140-11: Case containing one 5 mg/0.5 mL single-dose, pre-filled syringe
- NDC78670-140-02: Carton containing two cases, each of which contain one 5 mg/0.5 mL single-dose, pre-filled syringe
16.2 Storage and Handling
Store ZIMHI in the outer case provided.
Store at controlled room temperature 20°C to 25°C (68°F to 77°F), excursions between 15° and 30° (59° and 86° F) are allowed. Do not refrigerate. Protect from light, extreme heat, and freezing.
Prior to a medical emergency (during storage), periodically visually inspect ZIMHI through the viewing window on the device. If the solution is discolored yellow or brown color, cloudy, or contains particles, replace ZIMHI with a new one.
17. Patient Counseling Information
Advise the patient and family members or caregivers to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Instruct patients and their family members or caregivers to become familiar with all information contained in the case and carton as soon as they receive ZIMHI.
Inform patients of the following:
- ZIMHI is for the emergency treatment of known or suspected overdose.
- It is administered by a caregiver according to the Instructions for Use.
- After administration, the caregiver should get emergency help immediately.
INSTRUCTIONS FOR USE ZIMHI™ (ZIM-hye) (naloxone hydrochloride injection)
Pre-filled syringe
Use ZIMHI for known or suspected opioid overdose in adults and children.
ZIMHI is intended to be administered by people 12 years of age or older. Younger children or those with limited hand strength may find the device difficult to use.
Read this Instructions for Use carefully before you use this product.
Before you need to use your ZIMHI syringe make sure your healthcare provider shows you the right way to use it. Parents, caregivers, and others who may be in a position to administer ZIMHI should also understand how to use it. If you have questions, ask your healthcare provider.
Get Ready to Use ZIMHI
ZIMHI works like a standard pre-filled syringe.
ZIMHI is injected downwards, into the middle of the outer thigh, (as shown), through clothing if needed.
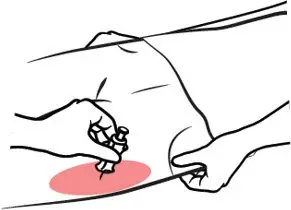
Ready to Use ZIMHI

Place the patient on their back.
When ready to inject, pull off cap to expose needle.

Do not put finger on top of the device.
In children under the age of 1 year old, pinch the thigh muscle while administering the dose.
Hold ZIMHI by finger grips only and slowly insert the needle into the thigh.
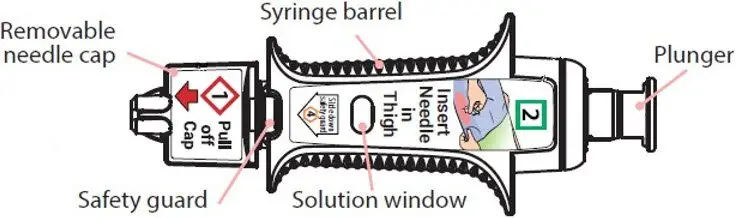

After needle is in thigh: Push the plunger all the way down until it clicks and hold for 2 seconds.

After Use
Right after the injection, using one hand with fingers behind the needle, slide the safety guard over the needle. Do not use two hands to activate the safety guard. Put the used syringe into the blue case and close the case.
 Get Help
Get Help
Get emergency medical help now

Tell healthcare provider that you gave an injection of naloxone hydrochloride.
Turn the patient on their side (recovery position) after giving ZIMHI.
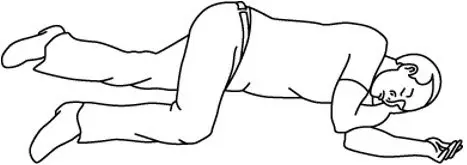
Use second syringe if needed

You may need a second ZIMHI syringe if symptoms continue or return.
If symptoms return after an injection with ZIMHI, an additional injection using another ZIMHI pre-filled syringe may be needed. Give additional injections using a new ZIMHI pre-filled syringe every 2 to 3 minutes and continue to closely watch the person until emergency help is received. Using ZIMHI does not take the place of emergency medical care.
After Use and Disposal
- ZIMHI is a single-dose pre-filled syringe that delivers a fixed dose of naloxone hydrochloride. The pre-filled syringe cannot be reused. It is normal for most of the medicine to remain in the syringe after the dose has been injected.
- The correct dose has been injected if the plunger has been pushed all the way down and the solution window is at least partially blocked.
- Tell the healthcare provider that you have received or administered an injection of naloxone hydrochloride. Show the healthcare provider where the injection was administered.
- Give your used ZIMHI syringe, contained in the blue case, to the healthcare provider for inspection and proper disposal.
For more information see Patient Information sheet or ask your healthcare provider.
How to Store — Keep ZIMHI in its plastic case nearby and ready for use at all times.
- Store at controlled room temperature, between 68°F to 77°F (20°C to 25°C). Do not expose to extreme cold or heat. For example, do not store in your vehicle's glove box and do not store in the refrigerator or freezer.
- Store the ZIMHI syringe in its closed plastic case to protect from light.
Check Your Stored ZIMHI Often - The solution should be clear when viewed through the window on the device. If the solution is discolored (yellowish or brown color), cloudy or contains particles, replace ZIMHI with a new one.
Your ZIMHI Has an Expiration Date — Example YYYY-MM. Replace ZIMHI before the last day of expiration month. Dispose of expired ZIMHI properly by taking ZIMHI in its case to a healthcare provider or hospital emergency room.
For more information about ZIMHI pre-filled syringe and proper use of ZIMHI, call 1-800-230-3935 or visit www.ZIMHI.com.
ZIMHI is a trademark of Adamis Pharmaceuticals Corporation.
Manufactured for Adamis Pharmaceuticals, San Diego, CA 92130, USA.
Distributed By USWM LLC, Louisville, KY 40241.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: October 2021
| ZIMHI
naloxone hydrochloride injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - USWM, LLC (117542566) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Alliance Medical Products, Inc (dba Siegfried Irvine) | 102688657 | MANUFACTURE(78670-140) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Avomeen Analytical Services | 965534816 | ANALYSIS(78670-140) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Phillips Medisize LLC | 037805210 | MANUFACTURE(78670-140) , LABEL(78670-140) , PACK(78670-140) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Noramco, LLC | 166506142 | API MANUFACTURE(78670-140) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eurofins Lancaster Laboratories, Inc. | 069777290 | ANALYSIS(78670-140) | |