Drug Detail:Aubagio (Teriflunomide [ ter-i-floo-noe-mide ])
Drug Class: Selective immunosuppressants
Highlights of Prescribing Information
AUBAGIO (teriflunomide) tablets, for oral use
Initial U.S. Approval: 2012
WARNING: HEPATOTOXICITY and EMBRYOFETAL TOXICITY
See full prescribing information for complete boxed warning.
- Hepatotoxicity
Clinically significant and potentially life-threatening liver injury, including acute liver failure requiring transplant, has been reported in patients treated with AUBAGIO in the postmarketing setting (5.1). Concomitant use of AUBAGIO with other hepatotoxic drugs may increase the risk of severe liver injury. Obtain transaminase and bilirubin levels within 6 months before initiation of AUBAGIO and monitor ALT levels at least monthly for six months (5.1). If drug induced liver injury is suspected, discontinue AUBAGIO and start accelerated elimination procedure (5.3).
- Embryofetal Toxicity
Teratogenicity and embryolethality occurred in animals administered teriflunomide (5.2, 8.1). Exclude pregnancy prior to initiating AUBAGIO therapy (4, 5.2, 8.1, 8.3). Advise use of effective contraception in females of reproductive potential during treatment and during an accelerated drug elimination procedure (4, 5.2, 5.3, 8.1, 8.3). Stop AUBAGIO and use an accelerated drug elimination procedure if the patient becomes pregnant (5.2, 5.3, 8.1).
Indications and Usage for Aubagio
AUBAGIO is a pyrimidine synthesis inhibitor indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults. (1)
Aubagio Dosage and Administration
7 mg or 14 mg orally once daily, with or without food. (2)
Dosage Forms and Strengths
7 mg and 14 mg film-coated tablets (3)
Contraindications
- Severe hepatic impairment (4, 5.1)
- Pregnancy (4, 5.2, 8.1)
- Hypersensitivity (4, 5.5)
- Current leflunomide treatment (4)
Warnings and Precautions
- Elimination of AUBAGIO can be accelerated by administration of cholestyramine or activated charcoal for 11 days. (5.3)
- AUBAGIO may decrease WBC. A recent CBC should be available before starting AUBAGIO. Monitor for signs and symptoms of infection. Consider suspending treatment with AUBAGIO in case of serious infection. Do not start AUBAGIO in patients with active infections. (5.4)
- Stop AUBAGIO if patient has anaphylaxis, angioedema, Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms; initiate rapid elimination. (5.3, 5.5, 5.6, 5.7)
- If patient develops symptoms consistent with peripheral neuropathy, evaluate patient and consider discontinuing AUBAGIO. (5.8)
- AUBAGIO may increase blood pressure. Measure blood pressure at treatment initiation and monitor blood pressure during treatment. (5.9)
Adverse Reactions/Side Effects
Most common adverse reactions (≥10% and ≥2% greater than placebo): headache, diarrhea, nausea, alopecia, increase in ALT. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Genzyme Corporation at 1-800-745-4447 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Drugs metabolized by CYP2C8 and OAT3 transporters: Monitor patients because teriflunomide may increase exposure of these drugs. (7)
- Teriflunomide may increase exposure of ethinylestradiol and levonorgestrel. Choose an appropriate oral contraceptive. (7)
- Drugs metabolized by CYP1A2: Monitor patients because teriflunomide may decrease exposure of these drugs. (7)
- Warfarin: Monitor INR as teriflunomide may decrease INR. (7)
- Drugs metabolized by BCRP and OATP1B1/B3 transporters: Monitor patients because teriflunomide may increase exposure of these drugs. (7)
- Rosuvastatin: The dose of rosuvastatin should not exceed 10 mg once daily in patients taking AUBAGIO. (7)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2022
Full Prescribing Information
WARNING: HEPATOTOXICITY and EMBRYOFETAL TOXICITY
- Hepatotoxicity
Clinically significant and potentially life-threatening liver injury, including acute liver failure requiring transplant, has been reported in patients treated with AUBAGIO in the postmarketing setting [see Warnings and Precautions (5.1)]. Concomitant use of AUBAGIO with other hepatotoxic drugs may increase the risk of severe liver injury.
Obtain transaminase and bilirubin levels within 6 months before initiation of AUBAGIO therapy. Monitor ALT levels at least monthly for six months after starting AUBAGIO [see Warnings and Precautions (5.1)]. If drug induced liver injury is suspected, discontinue AUBAGIO and start an accelerated elimination procedure with cholestyramine or charcoal [see Warnings and Precautions (5.3)]. AUBAGIO is contraindicated in patients with severe hepatic impairment [see Contraindications (4)]. Patients with pre-existing liver disease may be at increased risk of developing elevated serum transaminases when taking AUBAGIO.
- Embryofetal Toxicity
AUBAGIO is contraindicated for use in pregnant women and in females of reproductive potential who are not using effective contraception because of the potential for fetal harm. Teratogenicity and embryolethality occurred in animals at plasma teriflunomide exposures lower than that in humans. Exclude pregnancy before the start of treatment with AUBAGIO in females of reproductive potential. Advise females of reproductive potential to use effective contraception during AUBAGIO treatment and during an accelerated drug elimination procedure after AUBAGIO treatment. Stop AUBAGIO and use an accelerated drug elimination procedure if the patient becomes pregnant [see Contraindications (4), Warnings and Precautions (5.2, 5.3), Use in Specific Populations (8.1, 8.3), and Clinical Pharmacology (12.3)].
1. Indications and Usage for Aubagio
AUBAGIO® is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
2. Aubagio Dosage and Administration
The recommended dose of AUBAGIO is 7 mg or 14 mg orally once daily. AUBAGIO can be taken with or without food.
3. Dosage Forms and Strengths
AUBAGIO is available as 7 mg and 14 mg tablets.
The 14 mg tablet is a pale blue to pastel blue, pentagonal film-coated tablet with the dose strength "14" imprinted on one side and engraved with the corporate logo on the other side. Each tablet contains 14 mg of teriflunomide.
The 7 mg tablet is a very light greenish-bluish grey to pale greenish-blue, hexagonal film-coated tablet with the dose strength "7" imprinted on one side and engraved with the corporate logo on the other side. Each tablet contains 7 mg of teriflunomide.
5. Warnings and Precautions
5.1 Hepatotoxicity
Clinically significant and potentially life-threatening liver injury, including acute liver failure requiring transplant, has been reported in patients treated with AUBAGIO in the postmarketing setting. Patients with pre-existing liver disease and patients taking other hepatotoxic drugs may be at increased risk for developing liver injury when taking AUBAGIO. Clinically significant liver injury can occur at any time during treatment with AUBAGIO.
Patients with pre-existing acute or chronic liver disease, or those with serum alanine aminotransferase (ALT) greater than two times the upper limit of normal (ULN) before initiating treatment, should not normally be treated with AUBAGIO. AUBAGIO is contraindicated in patients with severe hepatic impairment [see Contraindications (4)].
In placebo-controlled trials in adult patients, ALT greater than three times the ULN occurred in 61/1045 (5.8%) and 62/1002 (6.2%) of patients receiving AUBAGIO 7 mg and 14 mg, respectively, and 38/997 (3.8%) of patients receiving placebo, during the treatment period. These elevations occurred mostly within the first year of treatment. Half of the cases returned to normal without drug discontinuation. In clinical trials, if ALT elevation was greater than three times the ULN on two consecutive tests, AUBAGIO was discontinued and patients underwent an accelerated elimination procedure [see Warnings and Precautions (5.3)]. Of the patients who underwent discontinuation and accelerated elimination in controlled trials, half returned to normal or near normal values within 2 months.
One patient in the controlled trials in adult patients developed ALT 32 times the ULN and jaundice 5 months after initiation of AUBAGIO 14 mg treatment. The patient was hospitalized for 5 weeks and recovered after plasmapheresis and cholestyramine accelerated elimination procedure. AUBAGIO-induced liver injury in this patient could not be ruled out.
Obtain serum transaminase and bilirubin levels within 6 months before initiation of AUBAGIO therapy. Monitor ALT levels at least monthly for six months after starting AUBAGIO. Consider additional monitoring when AUBAGIO is given with other potentially hepatotoxic drugs.
Consider discontinuing AUBAGIO if serum transaminase increase (greater than three times the ULN) is confirmed. Monitor serum transaminase and bilirubin on AUBAGIO therapy, particularly in patients who develop symptoms suggestive of hepatic dysfunction, such as unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or jaundice and/or dark urine. If liver injury is suspected to be AUBAGIO-induced, discontinue AUBAGIO and start an accelerated elimination procedure [see Warnings and Precautions (5.3)] and monitor liver tests weekly until normalized. If AUBAGIO-induced liver injury is unlikely because some other probable cause has been found, resumption of AUBAGIO therapy may be considered.
5.2 Embryofetal Toxicity
AUBAGIO may cause fetal harm when administered to a pregnant woman. Teratogenicity and embryofetal lethality occurred in animal reproduction studies in multiple animal species at plasma teriflunomide exposures similar to or lower than that in humans at the maximum recommended human dose (MRHD) of 14 mg/day [see Use in Specific Populations (8.1)].
AUBAGIO is contraindicated for use in pregnant women and in females of reproductive potential not using effective contraception [see Contraindications (4)]. Exclude pregnancy before starting treatment with AUBAGIO in females of reproductive potential [see Dosage and Administration (2)]. Advise females of reproductive potential to use effective contraception during AUBAGIO treatment and during an accelerated drug elimination procedure after AUBAGIO treatment [see Use in Specific Populations (8.3)]. If a woman becomes pregnant while taking AUBAGIO, stop treatment with AUBAGIO, apprise the patient of the potential risk to a fetus, and perform an accelerated drug elimination procedure to achieve a plasma teriflunomide concentration of less than 0.02 mg/L [see Warnings and Precautions (5.3)].
Upon discontinuing AUBAGIO, it is recommended that all females of reproductive potential undergo an accelerated drug elimination procedure. Women receiving AUBAGIO treatment who wish to become pregnant must discontinue AUBAGIO and undergo an accelerated drug elimination procedure, which includes verification that plasma concentrations of teriflunomide are less than 0.02 mg/L (0.02 mcg/mL). Men wishing to father a child should also discontinue use of AUBAGIO and either undergo an accelerated elimination procedure or wait until verification that the plasma teriflunomide concentration is less than 0.02 mg/L (0.02 mcg/mL) [see Use in Specific Populations (8.3)]. Based on animal data, human plasma concentrations of teriflunomide of less than 0.02 mg/L (0.02 mcg/mL) are expected to have minimal embryofetal risk [see Contraindications (4), Warnings and Precautions (5.3), and Use in Specific Populations (8.1)].
5.3 Procedure for Accelerated Elimination of Teriflunomide
Teriflunomide is eliminated slowly from the plasma [see Clinical Pharmacology (12.3)]. Without an accelerated elimination procedure, it takes on average 8 months to reach plasma concentrations less than 0.02 mg/L, although because of individual variations in drug clearance it may take as long as 2 years. An accelerated elimination procedure could be used at any time after discontinuation of AUBAGIO. Elimination can be accelerated by either of the following procedures:
- Administration of cholestyramine 8 g every 8 hours for 11 days. If cholestyramine 8 g three times a day is not well tolerated, cholestyramine 4 g three times a day can be used.
- Administration of 50 g oral activated charcoal powder every 12 hours for 11 days.
If either elimination procedure is poorly tolerated, treatment days do not need to be consecutive unless there is a need to lower teriflunomide plasma concentration rapidly.
At the end of 11 days, both regimens successfully accelerated teriflunomide elimination, leading to more than 98% decrease in teriflunomide plasma concentrations.
Use of the accelerated elimination procedure may potentially result in return of disease activity if the patient had been responding to AUBAGIO treatment.
5.4 Bone Marrow Effects/Immunosuppression Potential/Infections
5.5 Hypersensitivity Reactions
AUBAGIO can cause anaphylaxis and severe allergic reactions [see Contraindications (4)]. Signs and symptoms have included dyspnea, urticaria, and angioedema including lips, eyes, throat, and tongue.
Inform patients of the signs and symptoms of anaphylaxis and angioedema.
5.6 Serious Skin Reactions
Cases of serious skin reactions, sometimes fatal, including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) [see Warnings and Precautions (5.7)], have been reported with AUBAGIO. Fatal outcomes were reported in one case of TEN and one case of DRESS.
Inform patients of the signs and symptoms that may signal a serious skin reaction. Instruct patients to discontinue AUBAGIO and seek immediate medical care should these signs and symptoms occur. Unless the reaction is clearly not drug related, discontinue AUBAGIO and begin an accelerated elimination procedure immediately [see Warnings and Precautions (5.3)]. In such cases, patients should not be re-exposed to teriflunomide [see Contraindications (4)].
5.7 Drug Reaction with Eosinophilia and Systemic Symptoms
Drug reaction with eosinophilia and systemic symptoms (DRESS), also known as multiorgan hypersensitivity, has occurred with AUBAGIO. One fatal case of DRESS that occurred in close temporal association (34 days) with the initiation of AUBAGIO treatment has been reported in the postmarketing setting. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy and/or facial swelling, in association with other organ system involvement, such as hepatitis, nephritis, hematologic abnormalities, myocarditis, or myositis, sometimes resembling an acute viral infection. Eosinophilia is often present. This disorder is variable in its expression, and other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity (e.g., fever, lymphadenopathy) may be present even though rash is not evident. If such signs or symptoms are present, the patient should be evaluated immediately.
Discontinue AUBAGIO, unless an alternative etiology for the signs or symptoms is established, and begin an accelerated elimination procedure immediately [see Warnings and Precautions (5.3)]. In such cases, patients should not be re-exposed to teriflunomide [see Contraindications (4)].
5.8 Peripheral Neuropathy
In placebo-controlled studies in adult patients, peripheral neuropathy, including both polyneuropathy and mononeuropathy (e.g., carpal tunnel syndrome), occurred more frequently in patients taking AUBAGIO than in patients taking placebo. The incidence of peripheral neuropathy confirmed by nerve conduction studies was 1.4% (13 patients) and 1.9% (17 patients) of patients receiving 7 mg and 14 mg of AUBAGIO, respectively, compared with 0.4% receiving placebo (4 patients). Treatment was discontinued in 0.7% (8 patients) with confirmed peripheral neuropathy (3 patients receiving AUBAGIO 7 mg and 5 patients receiving AUBAGIO 14 mg). Five of them recovered following treatment discontinuation. Not all cases of peripheral neuropathy resolved with continued treatment. Peripheral neuropathy also occurred in patients receiving leflunomide.
Age older than 60 years, concomitant neurotoxic medications, and diabetes may increase the risk for peripheral neuropathy. If a patient taking AUBAGIO develops symptoms consistent with peripheral neuropathy, such as bilateral numbness or tingling of hands or feet, consider discontinuing AUBAGIO therapy and performing an accelerated elimination procedure [see Warnings and Precautions (5.3)].
5.9 Increased Blood Pressure
In placebo-controlled studies in adult patients, the mean change from baseline to the end of study in systolic blood pressure was +2.3 mmHg and +2.7 mmHg for AUBAGIO 7 mg and 14 mg, respectively, and -0.6 mmHg for placebo. The change from baseline in diastolic blood pressure was +1.4 mmHg and +1.9 mmHg for AUBAGIO 7 mg and 14 mg, respectively, and -0.3 mmHg for placebo. Hypertension was an adverse reaction in 3.1% and 4.3% of patients treated with 7 mg or 14 mg of AUBAGIO compared with 1.8% for placebo. Check blood pressure before start of AUBAGIO treatment and periodically thereafter. Elevated blood pressure should be appropriately managed during treatment with AUBAGIO.
5.10 Respiratory Effects
Interstitial lung disease, including acute interstitial pneumonitis, has been reported with AUBAGIO in the postmarketing setting.
Interstitial lung disease and worsening of pre-existing interstitial lung disease have been reported during treatment with leflunomide. Interstitial lung disease may be fatal and may occur acutely at any time during therapy with a variable clinical presentation. New onset or worsening pulmonary symptoms, such as cough and dyspnea, with or without associated fever, may be a reason for discontinuation of therapy and for further investigation as appropriate. If discontinuation of the drug is necessary, consider initiation of an accelerated elimination procedure [see Warnings and Precautions (5.3)].
5.11 Pancreatitis in Pediatric Patients
AUBAGIO is not approved for use in pediatric patients. In the pediatric clinical trial, cases of pancreatitis were observed in 1.8% (2/109) of patients receiving AUBAGIO; one of these cases was serious [see Use in Specific Populations (8.4)]. If pancreatitis is suspected, discontinue teriflunomide and start an accelerated elimination procedure [see Warnings and Precautions (5.3)].
5.12 Concomitant Use with Immunosuppressive or Immunomodulating Therapies
Coadministration with antineoplastic or immunosuppressive therapies used for treatment of multiple sclerosis has not been evaluated. Safety studies in which AUBAGIO was concomitantly administered with other immune modulating therapies for up to one year (interferon beta, glatiramer acetate) did not reveal any specific safety concerns. The long term safety of these combinations in the treatment of multiple sclerosis has not been established.
In any situation in which the decision is made to switch from AUBAGIO to another agent with a known potential for hematologic suppression, it would be prudent to monitor for hematologic toxicity, because there will be overlap of systemic exposure to both compounds. Use of an accelerated elimination procedure may decrease this risk, but may also potentially result in return of disease activity if the patient had been responding to AUBAGIO treatment [see Warnings and Precautions (5.3)].
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described elsewhere in the prescribing information:
- Hepatotoxicity [see Contraindications (4) and Warnings and Precautions (5.1)]
- Bone Marrow Effects/Immunosuppression Potential/Infections [see Warnings and Precautions (5.4)]
- Hypersensitivity Reactions [see Contraindications (4) and Warnings and Precautions (5.5)]
- Serious Skin Reactions [see Warnings and Precautions (5.6)]
- Drug Reaction with Eosinophilia and Systemic Symptoms [see Warnings and Precautions (5.7)]
- Peripheral Neuropathy [see Warnings and Precautions (5.8)]
- Increased Blood Pressure [see Warnings and Precautions (5.9)]
- Respiratory Effects [see Warnings and Precautions (5.10)]
- Pancreatitis in Pediatric Patients [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
A total of 2047 patients receiving AUBAGIO (7 mg or 14 mg once daily) constituted the safety population in the pooled analysis of placebo-controlled studies in patients with relapsing forms of multiple sclerosis; of these, 71% were female. The average age was 37 years.
Table 1 lists adverse reactions in placebo-controlled trials with rates that were at least 2% for AUBAGIO patients and also at least 2% above the rate in placebo patients. The most common were headache, an increase in ALT, diarrhea, alopecia, and nausea. The adverse reaction most commonly associated with discontinuation was an increase in ALT (3.3%, 2.6%, and 2.3% of all patients in the AUBAGIO 7 mg, AUBAGIO 14 mg, and placebo treatment arms, respectively).
| Adverse Reaction | AUBAGIO 7 mg (N=1045) | AUBAGIO 14 mg (N=1002) | Placebo (N=997) |
|---|---|---|---|
| Headache | 18% | 16% | 15% |
| Increase in Alanine aminotransferase | 13% | 15% | 9% |
| Diarrhea | 13% | 14% | 8% |
| Alopecia | 10% | 13% | 5% |
| Nausea | 8% | 11% | 7% |
| Paresthesia | 8% | 9% | 7% |
| Arthralgia | 8% | 6% | 5% |
| Neutropenia | 4% | 6% | 2% |
| Hypertension | 3% | 4% | 2% |
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of AUBAGIO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and Lymphatic System Disorders: Thrombocytopenia [see Warnings and Precautions (5.4)]
- Gastrointestinal Disorders: Pancreatitis, colitis
- Hepatobiliary Disorders: Drug-induced liver injury (DILI) [see Warnings and Precautions (5.1)]
- Immune System Disorders: Hypersensitivity reactions, some of which were severe, such as anaphylaxis and angioedema [see Warnings and Precautions (5.5)]
- Respiratory, Thoracic, and Mediastinal Disorders: Interstitial lung disease [see Warnings and Precautions (5.10)]
- Skin and Subcutaneous Tissue Disorders: Severe skin reactions, including toxic epidermal necrolysis and Stevens-Johnson syndrome [see Warnings and Precautions (5.6)]; drug reaction with eosinophilia and systemic symptoms (DRESS) [see Warnings and Precautions (5.7)]; psoriasis or worsening of psoriasis (including pustular psoriasis and nail psoriasis); nail disorders
8. Use In Specific Populations
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Effectiveness of AUBAGIO for the treatment of relapsing form of multiple sclerosis in pediatric patients (10 to 17 years of age) was not established in an adequate and well-controlled clinical study in 166 patients (109 patients received once-daily doses of AUBAGIO and 57 patients received placebo) for up to 96 weeks.
Pancreatitis has been reported in adults in the postmarketing setting, but appears to occur at higher frequency in the pediatric population. In this pediatric study, cases of pancreatitis were reported in 1.8% (2/109) of patients who received AUBAGIO compared to no patients in the placebo group. All patients in the pediatric trial recovered or were recovering after treatment discontinuation and accelerated elimination procedure [see Warnings and Precautions (5.11)].
Additionally, elevated or abnormal blood creatine phosphokinase was reported in 6.4% of pediatric patients who received AUBAGIO compared to no patients in the placebo group.
8.6 Hepatic Impairment
No dosage adjustment is necessary for patients with mild and moderate hepatic impairment. The pharmacokinetics of teriflunomide in severe hepatic impairment has not been evaluated. AUBAGIO is contraindicated in patients with severe hepatic impairment [see Contraindications (4), Warnings and Precautions (5.1), and Clinical Pharmacology (12.3)].
10. Overdosage
There is no experience regarding teriflunomide overdose or intoxication in humans. Teriflunomide 70 mg daily up to 14 days was well tolerated by healthy subjects.
In the event of clinically significant overdose or toxicity, cholestyramine or activated charcoal is recommended to accelerate elimination [see Warnings and Precautions (5.3)].
11. Aubagio Description
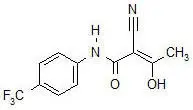
AUBAGIO (teriflunomide) is an oral de novo pyrimidine synthesis inhibitor of the DHO-DH enzyme, with the chemical name (Z)-2-Cyano-3-hydroxy-but-2-enoic acid-(4-trifluoromethylphenyl)-amide. Its molecular weight is 270.21, and the empirical formula is C12H9F3N2O2 with the following chemical structure:
Teriflunomide is a white to almost white powder that is sparingly soluble in acetone, slightly soluble in polyethylene glycol and ethanol, very slightly soluble in isopropanol and practically insoluble in water.
Teriflunomide is formulated as film-coated tablets for oral administration. AUBAGIO tablets contain 7 mg or 14 mg of teriflunomide and the following inactive ingredients: lactose monohydrate, corn starch, hydroxypropyl cellulose, microcrystalline cellulose, sodium starch glycolate, and magnesium stearate. The film coating for the 14 mg tablet is made of hypromellose, titanium dioxide, talc, polyethylene glycol and indigo carmine aluminum lake. In addition to these, the 7 mg tablet film coating includes iron oxide yellow.
12. Aubagio - Clinical Pharmacology
12.1 Mechanism of Action
Teriflunomide, an immunomodulatory agent with anti-inflammatory properties, inhibits dihydroorotate dehydrogenase, a mitochondrial enzyme involved in de novo pyrimidine synthesis. The exact mechanism by which teriflunomide exerts its therapeutic effect in multiple sclerosis is unknown but may involve a reduction in the number of activated lymphocytes in CNS.
12.3 Pharmacokinetics
Teriflunomide is the principal active metabolite of leflunomide and is responsible for leflunomide's activity in vivo. At recommended doses, teriflunomide and leflunomide result in a similar range of plasma concentrations of teriflunomide.
Based on a population analysis of teriflunomide in healthy adult volunteers and adult MS patients, median t1/2 was approximately 18 and 19 days after repeated doses of 7 mg and 14 mg respectively. It takes approximately 3 months respectively to reach steady-state concentrations. The estimated AUC accumulation ratio is approximately 30 after repeated doses of 7 or 14 mg.
Drug Interaction Studies
Teriflunomide is not metabolized by Cytochrome P450 or flavin monoamine oxidase enzymes.
14. Clinical Studies
Four randomized, controlled, double-blind clinical trials established the efficacy of AUBAGIO in patients with relapsing forms of multiple sclerosis.
Study 1 was a double-blind, placebo-controlled clinical trial that evaluated once daily doses of AUBAGIO 7 mg and AUBAGIO 14 mg for up to 26 months in patients with relapsing forms of multiple sclerosis. Patients were required to have a diagnosis of multiple sclerosis exhibiting a relapsing clinical course, with or without progression, and to have experienced at least one relapse over the year preceding the trial or at least two relapses over the two years preceding the trial. Patients were required not to have received interferon-beta for at least four months, or any other multiple sclerosis medication for at least six months before entering the study, nor were these medications permitted during the study. Neurological evaluations were to be performed at screening, every 12 weeks until week 108, and after suspected relapses. MRI was to be performed at screening, and at week 24, 48, 72, and 108. The primary endpoint was the annualized relapse rate (ARR).
In Study 1, 1088 patients were randomized to receive AUBAGIO 7 mg (n=366), AUBAGIO 14 mg (n=359), or placebo (n=363). At entry, patients had an Expanded Disability Status Scale (EDSS) score ≤5.5. Patients had a mean age of 38 years, mean disease duration of 5 years, and mean EDSS at baseline of 2.7. A total of 91% of patients had relapsing remitting multiple sclerosis, and 9% had a progressive form of multiple sclerosis with relapses. The mean duration of treatment was 635, 627, and 631 days for AUBAGIO 7 mg, AUBAGIO 14 mg, and placebo, respectively. The percentage of patients who completed the study treatment period was 75%, 73%, and 71% for AUBAGIO 7 mg, AUBAGIO 14 mg, and placebo, respectively.
There was a statistically significant reduction in ARR for patients who received AUBAGIO 7 mg or AUBAGIO 14 mg, compared to patients who received placebo (see Table 2). There was a consistent reduction of the ARR noted in subgroups defined by sex, age group, prior multiple sclerosis therapy, and baseline disease activity.
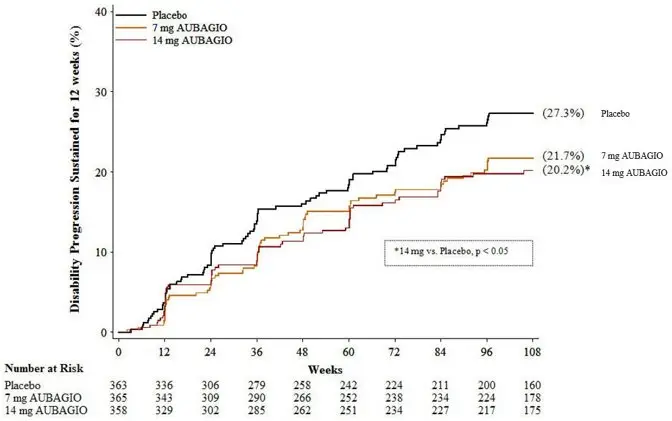
There was a statistically significant reduction in the relative risk of disability progression at week 108 sustained for 12 weeks (as measured by at least a 1-point increase from baseline EDSS ≤5.5 or a 0.5 point increase for those with a baseline EDSS >5.5) in the AUBAGIO 14 mg group compared to placebo (see Table 2 and Figure 1).
The effect of AUBAGIO on several magnetic resonance imaging (MRI) variables, including the total lesion volume of T2 and hypointense T1 lesions, was assessed in Study 1. The change in total lesion volume from baseline was significantly lower in the AUBAGIO 7 mg and AUBAGIO 14 mg groups than in the placebo group. Patients in both AUBAGIO groups had significantly fewer gadolinium-enhancing lesions per T1-weighted scan than those in the placebo group (see Table 2).
| AUBAGIO 7 mg N=365 | AUBAGIO 14 mg N=358 | Placebo N=363 |
|
|---|---|---|---|
|
|||
| Clinical Endpoints | |||
| Annualized relapse rate | 0.370 (p=0.0002) | 0.369 (p=0.0005) | 0.539 |
| Relative risk reduction | 31% | 31% | – |
| Percent of patients remaining relapse-free at week 108 | 53.7% | 56.5% | 45.6% |
| Percent disability progression at week 108 | 21.7% (p=0.084) | 20.2% (p=0.028) | 27.3% |
| Hazard ratio | 0.76 | 0.70 | – |
| MRI Endpoints | |||
| Median change from baseline in Total lesion volume* (mL) at week 108 | 0.755 (p=0.0317)† | 0.345 (p=0.0003)† | 1.127 |
| Mean number of Gd-enhancing T1-lesions per scan | 0.570 (p<0.0001) | 0.261 (p<0.0001) | 1.331 |
| Figure 1: Kaplan-Meier Plot of Time to Disability Progression Sustained for 12 Weeks (Study 1) |
|
|
Study 2 was a double-blind, placebo-controlled clinical trial that evaluated once daily doses of AUBAGIO 7 mg and AUBAGIO 14 mg for up to 40 months in patients with relapsing forms of multiple sclerosis. Patients were required to have a diagnosis of multiple sclerosis exhibiting a relapsing clinical course and to have experienced at least one relapse over the year preceding the trial, or at least two relapses over the two years preceding the trial. Patients were required not to have received any multiple sclerosis medication for at least three months before entering the trial, nor were these medications permitted during the trial. Neurological evaluations were to be performed at screening, every 12 weeks until completion, and after every suspected relapse. The primary end point was the ARR.
A total of 1165 patients received AUBAGIO 7 mg (n=407), AUBAGIO 14 mg (n=370), or placebo (n=388). Patients had a mean age of 38 years, a mean disease duration of 5 years, and a mean EDSS at baseline of 2.7. A total of 98% of patients had relapsing remitting multiple sclerosis, and 2% had a progressive form of multiple sclerosis with relapses. The mean duration of treatment was 552, 567, and 571 days for AUBAGIO 7 mg, AUBAGIO 14 mg, and placebo, respectively. The percentage of patients who completed the study treatment period was 67%, 66%, and 68% for AUBAGIO 7 mg, AUBAGIO 14 mg, and placebo, respectively.
There was a statistically significant reduction in the ARR for patients who received AUBAGIO 7 mg or AUBAGIO 14 mg compared to patients who received placebo (see Table 3). There was a consistent reduction of the ARR noted in subgroups defined by sex, age group, prior multiple sclerosis therapy, and baseline disease activity.
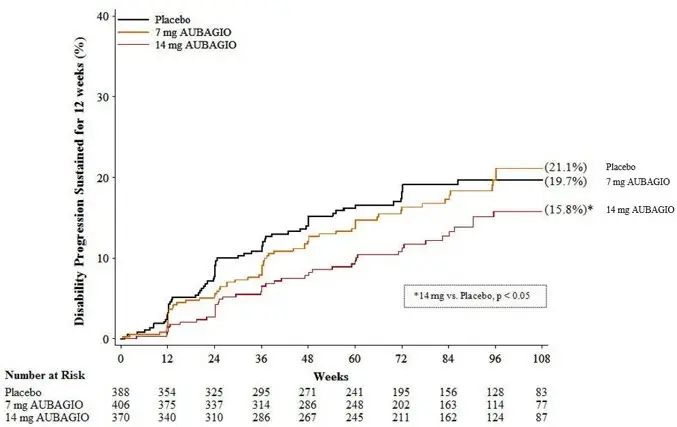
There was a statistically significant reduction in the relative risk of disability progression at week 108 sustained for 12 weeks (as measured by at least a 1-point increase from baseline EDSS ≤5.5 or a 0.5 point increase for those with a baseline EDSS >5.5) in the AUBAGIO 14 mg group compared to placebo (see Table 3 and Figure 2).
| AUBAGIO 7 mg N=407 | AUBAGIO 14 mg N=370 | Placebo N=388 |
|
|---|---|---|---|
| Clinical Endpoints | |||
| Annualized relapse rate | 0.389 (p=0.0183) | 0.319 (p=0.0001) | 0.501 |
| Relative risk reduction | 22% | 36% | – |
| Percent of patients remaining relapse-free at week 108 | 58.2% | 57.1% | 46.8% |
| Percent disability progression at week 108 | 21.1% (p=0.762) | 15.8% (p=0.044) | 19.7% |
| Hazard ratio | 0.96 | 0.69 | – |
| Figure 2: Kaplan-Meier Plot of Time to Disability Progression Sustained for 12 Weeks (Study 2) |
|
|
Study 3 was a double-blind, placebo-controlled clinical trial that evaluated once daily doses of AUBAGIO 7 mg and AUBAGIO 14 mg for up to 108 weeks in patients with relapsing multiple sclerosis. Patients were required to have had a first clinical event consistent with acute demyelination occurring within 90 days of randomization with 2 or more T2 lesions at least 3 mm in diameter that were characteristic of multiple sclerosis. A total of 614 patients received AUBAGIO 7 mg (n=203), AUBAGIO 14 mg (n=214), or placebo (n=197). Patients had a mean age of 32 years, EDSS at baseline of 1.7, and mean disease duration of two months. The proportion of patients free of relapse was greater in the AUBAGIO 7 mg (70.5%, p<0.05) and AUBAGIO 14 mg (72.2%, p<0.05) groups than in the placebo group (61.7%).
The effect of AUBAGIO on MRI activity was also demonstrated in Study 4, a randomized, double-blind, placebo-controlled clinical trial of multiple sclerosis patients with relapse. In Study 4, MRI was to be performed at baseline, 6 weeks, 12 weeks, 18 weeks, 24 weeks, 30 weeks, and 36 weeks after treatment initiation. A total of 179 patients were randomized to AUBAGIO 7 mg (n=61), AUBAGIO 14 mg (n=57), or placebo (n=61). Baseline demographics were consistent across treatment groups. The primary endpoint was the average number of unique active lesions/MRI scan during treatment. The mean number of unique active lesions per brain MRI scan during the 36-week treatment period was lower in patients treated with AUBAGIO 7 mg (1.06) and AUBAGIO 14 mg (0.98) as compared to placebo (2.69), the difference being statistically significant for both (p=0.0234 and p=0.0052, respectively).
16. How is Aubagio supplied
AUBAGIO is available as 7 mg and 14 mg tablets.
The 14 mg tablet is pale blue to pastel blue, pentagonal film-coated tablet with dose strength "14" imprinted on one side and engraved with corporate logo on the other side. Each tablet contains 14 mg of teriflunomide.
The 7 mg tablet is very light greenish-bluish grey to pale greenish-blue, hexagonal film-coated tablet with dose strength "7" imprinted on one side and engraved with corporate logo on the other side. Each tablet contains 7 mg of teriflunomide.
AUBAGIO 14 mg tablets are supplied as:
| NDC 58468-0210-4 | Carton containing a bottle of 30 tablets |
| NDC 58468-0210-1 | Carton of 5 tablets with one blister card with five tablets |
AUBAGIO 7 mg tablets are supplied as:
| NDC 58468-0211-4 | Carton containing a bottle of 30 tablets |
| NDC 58468-0211-2 | Carton of 5 tablets with one blister card with five tablets |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
A Medication Guide is required for distribution with AUBAGIO.
| AUBAGIO
teriflunomide tablet, film coated |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| AUBAGIO
teriflunomide tablet, film coated |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Genzyme Corporation (025322157) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| OPELLA HEALTHCARE INTERNATIONAL SAS | 275857012 | ANALYSIS(58468-0210, 58468-0211) , MANUFACTURE(58468-0210, 58468-0211) , PACK(58468-0210, 58468-0211) , LABEL(58468-0210, 58468-0211) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi-Aventis Deutschland GmbH | 313218430 | ANALYSIS(58468-0210, 58468-0211) , API MANUFACTURE(58468-0210, 58468-0211) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Micro-Macinazione SA | 480918515 | API MANUFACTURE(58468-0210, 58468-0211) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AndersonBrecon Inc. | 053217022 | LABEL(58468-0210, 58468-0211) , PACK(58468-0210, 58468-0211) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Penn Pharmaceutical Services Limited | 226277259 | ANALYSIS(58468-0210, 58468-0211) , MANUFACTURE(58468-0210, 58468-0211) , PACK(58468-0210, 58468-0211) , LABEL(58468-0210, 58468-0211) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| EUROAPI FRANCE | 276495414 | API MANUFACTURE(58468-0210, 58468-0211) | |