Drug Detail:Razadyne (Galantamine [ ga-lan-ta-meen ])
Drug Class: Cholinesterase inhibitors
Highlights of Prescribing Information
RAZADYNE® ER (galantamine extended-release capsules), for oral use
Initial U.S. Approval: 2001
Indications and Usage for Razadyne
RAZADYNE ER is a cholinesterase inhibitor indicated for the treatment of mild to moderate dementia of the Alzheimer's type (1)
Razadyne Dosage and Administration
- Recommended starting dosage is 8 mg/day in morning; increase to initial maintenance dose of 16 mg/day after a minimum of 4 weeks. Based on clinical benefit and tolerability, dosage may be increased to 24 mg/day after a minimum of 4 weeks at 16 mg/day. (2.1)
- Take with food; ensure adequate fluid intake during treatment (2.1)
- Hepatic impairment: should not exceed 16 mg/day for moderate hepatic impairment; do not use in patients with severe hepatic impairment (2.2)
- Renal impairment: should not exceed 16 mg/day for creatinine clearance 9 to 59 mL/min; do not use in patients with creatinine clearance less than 9 mL/min. (2.3)
- Conversion from galantamine tablets to RAZADYNE ER should occur at the same daily dosage with the last dose of galantamine tablets taken in evening and starting RAZADYNE ER once daily treatment the next morning. (2.5)
Dosage Forms and Strengths
Extended-release capsules: 8 mg, 16 mg, 24 mg (3)
Contraindications
Known hypersensitivity to galantamine hydrobromide or any excipients (4)
Warnings and Precautions
- Serious skin reactions: discontinue at first appearance of skin rash (5.1)
- All patients should be considered at risk for adverse effects on cardiac conduction, including bradycardia and AV block, due to vagotonic effects on sinoatrial and atrioventricular nodes (5.3)
- Active or occult gastrointestinal bleeding: monitor, especially those with an increased risk for developing ulcers (5.4)
- Cholinomimetics may cause bladder outflow obstruction (5.5)
- Monitor for respiratory adverse events in patients with a history of severe asthma or obstructive pulmonary disease (5.7)
Adverse Reactions/Side Effects
The most common adverse reactions (≥5%) were nausea, vomiting, diarrhea, dizziness, headache, and decreased appetite (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Pharmaceuticals, Inc. at 1-800-526-7736 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Potential to interfere with the activity of anticholinergic medications (7.1)
- Synergistic effect expected when given concurrently with succinylcholine, other cholinesterase inhibitors, similar neuromuscular blocking agents, or cholinergic agonists (7.2)
Use In Specific Populations
Pregnancy: Based on animal data may cause fetal harm. (8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2022
Related/similar drugs
donepezil, memantine, Aricept, Namenda, rivastigmine, ExelonFull Prescribing Information
1. Indications and Usage for Razadyne
RAZADYNE ER is indicated for the treatment of mild to moderate dementia of the Alzheimer's type.
2. Razadyne Dosage and Administration
2.1 Recommended Dosage and Administration
Administer RAZADYNE ER extended-release capsules once daily in the morning, preferably with food. Ensure adequate fluid intake during treatment.
The recommended starting dosage of RAZADYNE ER is 8 mg/day. Increase to the initial maintenance dosage of 16 mg/day after a minimum of 4 weeks. A further increase to 24 mg/day may be attempted after a minimum of 4 weeks at 16 mg/day. Increase dosage based upon assessment of clinical benefit and tolerability of the previous dosage.
The dosage of RAZADYNE ER shown to be effective in a controlled clinical trial is 16–24 mg/day.
2.2 Dosage in Patients with Hepatic Impairment
In patients with moderate hepatic impairment (Child-Pugh score of 7–9), the dosage should generally not exceed 16 mg/day. The use of RAZADYNE ER in patients with severe hepatic impairment (Child-Pugh score of 10–15) is not recommended [see Clinical Pharmacology (12.3)].
2.3 Dosage in Patients with Renal Impairment
In patients with creatinine clearance of 9 to 59 mL/min, the dosage should generally not exceed 16 mg/day. In patients with creatinine clearance less than 9 mL/min, the use of RAZADYNE ER is not recommended [see Clinical Pharmacology (12.3)].
2.4 Treatment Interruption
If therapy has been interrupted for more than three days, the patient should be restarted at the lowest dosage and the dosage escalated to the current dose.
The abrupt withdrawal of RAZADYNE ER in those patients who had been receiving dosages in the effective range was not associated with an increased frequency of adverse events in comparison with those continuing to receive the same dosages of that drug.
2.5 Switching to RAZADYNE ER from Galantamine Tablets
Patients currently being treated with galantamine tablets can convert to RAZADYNE ER (extended-release capsules) by taking their last dose of galantamine tablets in the evening and starting RAZADYNE ER once daily treatment the next morning. Converting from galantamine tablets to RAZADYNE ER should occur at the same total daily dosage.
3. Dosage Forms and Strengths
RAZADYNE ER extended-release capsules contain white to off-white pellets and are available in the following strengths:
- 8 mg white opaque, size 4 hard gelatin capsule with the inscription "GAL 8"
- 16 mg pink opaque, size 2 hard gelatin capsule with the inscription "GAL 16"
- 24 mg caramel opaque, size 1 hard gelatin capsule with the inscription "GAL 24"
4. Contraindications
RAZADYNE ER is contraindicated in patients with known hypersensitivity to galantamine hydrobromide or to any excipients used in the formulation.
5. Warnings and Precautions
5.1 Serious Skin Reactions
Serious skin reactions (Stevens-Johnson syndrome and acute generalized exanthematous pustulosis) have been reported in patients receiving RAZADYNE ER and galantamine tablets. Inform patients and caregivers that the use of RAZADYNE ER should be discontinued at the first appearance of a skin rash, unless the rash is clearly not drug-related. If signs or symptoms suggest a serious skin reaction, use of this drug should not be resumed and alternative therapy should be considered.
5.2 Anesthesia
Galantamine, as a cholinesterase inhibitor, is likely to exaggerate the neuromuscular blocking effects of succinylcholine-type and similar neuromuscular blocking agents during anesthesia.
5.3 Cardiovascular Conditions
Because of their pharmacological action, cholinesterase inhibitors have vagotonic effects on the sinoatrial and atrioventricular nodes, leading to bradycardia and AV block. Bradycardia and all types of heart block have been reported in patients both with and without known underlying cardiac conduction abnormalities [see Adverse Reactions (6.1, 6.2)]. Therefore, all patients should be considered at risk for adverse effects on cardiac conduction.
Patients treated with galantamine up to 24 mg/day using the recommended dosing schedule showed a dose-related increase in risk of syncope (placebo 0.7% [2/286]; 4 mg twice daily 0.4% [3/692]; 8 mg twice daily 1.3% [7/552]; 12 mg twice daily 2.2% [6/273]).
5.4 Gastrointestinal Conditions
Through their primary action, cholinomimetics may be expected to increase gastric acid secretion due to increased cholinergic activity. Therefore, patients should be monitored closely for symptoms of active or occult gastrointestinal bleeding, especially those with an increased risk for developing ulcers, e.g., those with a history of ulcer disease or patients using concurrent nonsteroidal anti-inflammatory drugs (NSAIDs). Clinical studies of galantamine have shown no increase, relative to placebo, in the incidence of either peptic ulcer disease or gastrointestinal bleeding.
Galantamine, as a predictable consequence of its pharmacological properties, has been shown to produce nausea, vomiting, diarrhea, anorexia, and weight loss. During therapy with RAZADYNE ER, the patient's weight should be monitored.
5.5 Genitourinary Conditions
Although this was not observed in clinical trials with galantamine, cholinomimetics may cause bladder outflow obstruction.
5.6 Neurological Conditions
Cholinesterase inhibitors are believed to have some potential to cause generalized convulsions [see Adverse Reactions (6.2)]. Seizure activity may also be a manifestation of Alzheimer's disease. Patients with Alzheimer's disease should be monitored closely for seizures while taking RAZADYNE ER.
An increase in cholinergic tone may worsen symptoms related to extrapyramidal disorders [see Adverse Reactions (6.2)].
5.7 Pulmonary Conditions
Because of its cholinomimetic action, RAZADYNE ER should be prescribed with care to patients with a history of severe asthma or obstructive pulmonary disease. Respiratory function should be monitored closely for the occurrence of respiratory adverse effects.
5.8 Deaths in Patients with Mild Cognitive Impairment (MCI)
In two randomized placebo-controlled trials of 2 years duration in patients with mild cognitive impairment (MCI), a total of 13 patients on galantamine (n=1026) and 1 patient on placebo (n=1022) died. The deaths were due to various causes which could be expected in an elderly population; about half of the galantamine deaths appeared to result from various vascular causes (myocardial infarction, stroke, and sudden death).
Although the difference in mortality between galantamine- and placebo-treated groups in these two studies was significant, the results are highly discrepant with other studies of galantamine. Specifically, in these two MCI studies, the mortality rate in the placebo-treated patients was markedly lower than the rate in placebo-treated patients in trials of galantamine in Alzheimer's disease or other dementias (0.7 per 1000 person years compared to 22–61 per 1000 person years, respectively). Although the mortality rate in the galantamine-treated MCI patients was also lower than that observed in galantamine-treated patients in Alzheimer's disease and other dementia trials (10.2 per 1000 person years compared to 23–31 per 1000 person years, respectively), the relative difference was much less. When the Alzheimer's disease and other dementia studies were pooled (n=6000), the mortality rate in the placebo group numerically exceeded that in the galantamine group. Furthermore, in the MCI studies, no patients in the placebo group died after 6 months, a highly unexpected finding in this population.
Individuals with mild cognitive impairment demonstrate isolated memory impairment greater than expected for their age and education, but do not meet current diagnostic criteria for Alzheimer's disease.
6. Adverse Reactions/Side Effects
Serious adverse reactions are discussed in more detail in the following sections of the labeling:
- Serious Skin Reactions [see Warnings and Precautions (5.1)]
- Cardiovascular Conditions [see Warnings and Precautions (5.3)]
- Gastrointestinal Conditions [see Warnings and Precautions (5.4)]
- Genitourinary Conditions [see Warnings and Precautions (5.5)]
- Neurological Conditions [see Warnings and Precautions (5.6)]
- Pulmonary Conditions [see Warnings and Precautions (5.7)]
- Deaths in Patients with Mild Cognitive Impairment (MCI) [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common adverse reactions (≥5%) in galantamine-treated patients from double-blind clinical trials were nausea, vomiting, diarrhea, dizziness, headache, and decreased appetite.
The most common adverse reactions associated with discontinuation (≥1%) in galantamine-treated patients from double-blind clinical trials were nausea (6.2%), vomiting (3.3%), decreased appetite (1.5%), and dizziness (1.3%).
The safety of the extended-release capsule and immediate-release tablet formulations of galantamine was evaluated in 3956 galantamine-treated patients who participated in 8 placebo-controlled clinical studies and 1454 patients in 5 open-label clinical studies with mild to moderate dementia of the Alzheimer's type. In clinical studies, the safety profile of once-daily treatment with extended-release galantamine was similar in frequency and nature to that seen with tablets. The information presented in this section was derived from pooled double-blind studies and from pooled open-label data.
Commonly-Observed Adverse Reactions in Double-Blind, Placebo-Controlled Clinical Trials
Table 1 lists the adverse reactions reported in ≥1% of galantamine-treated patients in 8 placebo-controlled, double-blind clinical trials.
| System/Organ Class Adverse Reaction | Galantamine (n=3956) % | Placebo (n=2546) % |
|---|---|---|
| Metabolism and Nutrition Disorders | ||
| Decreased appetite | 7.4 | 2.1 |
| Psychiatric Disorders | ||
| Depression | 3.6 | 2.3 |
| Nervous System Disorders | ||
| Dizziness | 7.5 | 3.4 |
| Headache | 7.1 | 5.5 |
| Tremor | 1.6 | 0.7 |
| Somnolence | 1.5 | 0.8 |
| Syncope | 1.4 | 0.6 |
| Lethargy | 1.3 | 0.4 |
| Cardiac Disorders | ||
| Bradycardia | 1.0 | 0.3 |
| Gastrointestinal Disorders | ||
| Nausea | 20.7 | 5.5 |
| Vomiting | 10.5 | 2.3 |
| Diarrhea | 7.4 | 4.9 |
| Abdominal pain | 3.8 | 2.0 |
| Abdominal discomfort | 2.1 | 0.7 |
| Dyspepsia | 1.5 | 1.0 |
| Musculoskeletal and Connective Tissue Disorders | ||
| Muscle spasms | 1.2 | 0.5 |
| General Disorders and Administration Site Conditions | ||
| Fatigue | 3.5 | 1.8 |
| Asthenia | 2.0 | 1.5 |
| Malaise | 1.1 | 0.5 |
| Investigations | ||
| Decreased weight | 4.7 | 1.5 |
| Injury, Poisoning and Procedural Complications | ||
| Fall | 3.9 | 3.0 |
| Laceration | 1.1 | 0.5 |
The majority of these adverse reactions occurred during the dose-escalation period. In those patients who experienced the most frequent adverse reaction, nausea, the median duration of the nausea was 5–7 days.
6.2 Postmarketing Experience
The following additional adverse reactions have been identified during post-approval use of RAZADYNE ER or galantamine tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
Immune System Disorders: Hypersensitivity
Psychiatric Disorders: Hallucinations
Nervous System Disorders: Seizures, extrapyramidal disorder [see Warnings and Precautions (5.6)]
Ear and Labyrinth Disorders: Tinnitus
Cardiac Disorders: Complete atrioventricular block
Vascular Disorders: Hypertension
Hepatobiliary Disorders: Hepatitis, increased hepatic enzyme
Skin and Subcutaneous Tissue Disorders: Stevens-Johnson syndrome, acute generalized exanthematous pustulosis, erythema multiforme
7. Drug Interactions
7.1 Use with Anticholinergics
Galantamine has the potential to interfere with the activity of anticholinergic medications [see Clinical Pharmacology (12.3)].
7.2 Use with Cholinomimetics and Other Cholinesterase Inhibitors
A synergistic effect is expected when cholinesterase inhibitors are given concurrently with succinylcholine, other cholinesterase inhibitors, similar neuromuscular blocking agents or cholinergic agonists such as bethanechol [see Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.5 Geriatric Use
Eight double-blind, placebo-controlled clinical trials and 5 open-label trials in a total of 6519 patients have investigated RAZADYNE ER and galantamine tablets in the treatment of mild to moderate dementia of the Alzheimer's type [see Adverse Reactions (6.1) and Clinical Studies (14)]. The mean age of patients enrolled in these clinical studies was 75 years; 78% of these patients were between 65 and 84 years of age, and 10% of patients were 85 years of age or older.
10. Overdosage
Because strategies for the management of overdose are continually evolving, it is advisable to contact a poison control center to determine the latest recommendations for the management of an overdose of any drug.
As in any case of overdose, general supportive measures should be utilized. Signs and symptoms of significant overdosing of galantamine are predicted to be similar to those of overdosing of other cholinomimetics. These effects generally involve the central nervous system, the parasympathetic nervous system, and the neuromuscular junction. In addition to muscle weakness or fasciculations, some or all of the following signs of cholinergic crisis may develop: severe nausea, vomiting, gastrointestinal cramping, salivation, lacrimation, urination, defecation, sweating, bradycardia, hypotension, respiratory depression, collapse and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved.
Tertiary anticholinergics such as atropine may be used as an antidote for RAZADYNE ER overdosage. Intravenous atropine sulfate titrated to effect is recommended at an initial dose of 0.5 to 1.0 mg i.v. with subsequent doses based upon clinical response. Atypical responses in blood pressure and heart rate have been reported with other cholinomimetics when co-administered with quaternary anticholinergics. It is not known whether galantamine and/or its metabolites can be removed by dialysis (hemodialysis, peritoneal dialysis, or hemofiltration). Dose-related signs of toxicity in animals included hypoactivity, tremors, clonic convulsions, salivation, lacrimation, chromodacryorrhea, mucoid feces, and dyspnea.
In one postmarketing report, one patient who had been taking 4 mg of galantamine daily for a week inadvertently ingested eight 4 mg tablets (32 mg total) on a single day. Subsequently, she developed bradycardia, QT prolongation, ventricular tachycardia and torsades de pointes accompanied by a brief loss of consciousness for which she required hospital treatment. Two additional cases of accidental ingestion of 32 mg (nausea, vomiting, and dry mouth; nausea, vomiting, and substernal chest pain) and one of 40 mg (vomiting), resulted in brief hospitalizations for observation with full recovery. One patient, who was prescribed 24 mg/day and had a history of hallucinations over the previous two years, mistakenly received 24 mg twice daily for 34 days and developed hallucinations requiring hospitalization. Another patient, who was prescribed 16 mg/day of oral solution, inadvertently ingested 160 mg (40 mL) and experienced sweating, vomiting, bradycardia, and near-syncope one hour later, which necessitated hospital treatment. His symptoms resolved within 24 hours.
11. Razadyne Description
RAZADYNE® ER (galantamine extended-release capsules) contains galantamine, a reversible, competitive acetylcholinesterase inhibitor, as the hydrobromide salt. Galantamine hydrobromide is known chemically as (4aS,6R,8aS)-4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-6H-benzofuro[3a,3,2-ef][2]benzazepin-6-ol hydrobromide. It has an empirical formula of C17H21NO3 •HBr and a molecular weight of 368.27. Galantamine hydrobromide is a white to almost white powder and is sparingly soluble in water. The structural formula for galantamine hydrobromide is:
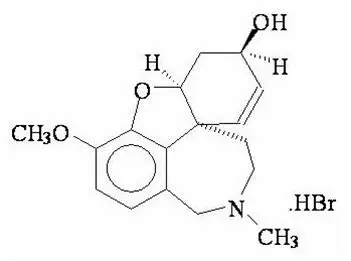
RAZADYNE ER extended-release capsules contain 8 mg, 16 mg, and 24 mg galantamine as 10.25 mg, 20.51 mg, and 30.76 mg of galantamine hydrobromide, respectively. Inactive ingredients include diethyl phthalate, ethylcellulose, gelatin, hypromellose, polyethylene glycol, sugar spheres (sucrose and starch), and titanium dioxide. The 16 mg capsule also contains red ferric oxide. The 24 mg capsule also contains red ferric oxide and yellow ferric oxide.
12. Razadyne - Clinical Pharmacology
12.1 Mechanism of Action
Although the etiology of cognitive impairment in Alzheimer's disease (AD) is not fully understood, it has been reported that acetylcholine-producing neurons degenerate in the brains of patients with Alzheimer's disease. The degree of this cholinergic loss has been correlated with degree of cognitive impairment and density of amyloid plaques (a neuropathological hallmark of Alzheimer's disease).
Galantamine, a tertiary alkaloid, is a competitive and reversible inhibitor of acetylcholinesterase. While the precise mechanism of galantamine's action is unknown, it is postulated to exert its therapeutic effect by enhancing cholinergic function. This is accomplished by increasing the concentration of acetylcholine through reversible inhibition of its hydrolysis by cholinesterase. If this mechanism is correct, galantamine's effect may lessen as the disease process advances and fewer cholinergic neurons remain functionally intact. There is no evidence that galantamine alters the course of the underlying dementing process.
12.3 Pharmacokinetics
The pharmacokinetics of galantamine are linear over a dose range of 8–32 mg/day.
14. Clinical Studies
The effectiveness of galantamine as a treatment for Alzheimer's disease is demonstrated by the results of 5 randomized, double-blind, placebo-controlled clinical investigations in patients with probable Alzheimer's disease, 4 with the immediate-release tablet and 1 with the extended-release capsule [diagnosed by NINCDS-ADRDA criteria, with Mini-Mental State Examination scores that were ≥10 and ≤24]. Doses studied with the tablet formulation were 8–32 mg/day given as twice daily doses. In 3 of the 4 studies with the tablet, patients were started on a low dose of 8 mg, then titrated weekly by 8 mg/day to 24 or 32 mg as assigned. In the fourth study (USA 4-week Dose Escalation Fixed-Dose Study) dose escalation of 8 mg/day occurred over 4-week intervals. The mean age of patients participating in these 4 galantamine trials was 75 years with a range of 41 to 100. Approximately 62% of patients were women and 38% were men. The racial distribution was White 94%, Black 3% and other races 3%. Two other studies examined a three times daily dosing regimen; these also showed or suggested benefit but did not suggest an advantage over twice daily dosing.
14.1 Study Outcome Measures
In each study, the primary effectiveness of galantamine was evaluated using a dual outcome assessment strategy as measured by the Alzheimer's Disease Assessment Scale (ADAS-cog) and the Clinician's Interview Based Impression of Change that required the use of caregiver information (CIBIC-plus).
The ability of galantamine to improve cognitive performance was assessed with the cognitive sub-scale of the Alzheimer's Disease Assessment Scale (ADAS-cog), a multi-item instrument that has been extensively validated in longitudinal cohorts of Alzheimer's disease patients. The ADAS-cog examines selected aspects of cognitive performance including elements of memory, orientation, attention, reasoning, language and praxis. The ADAS-cog scoring range is from 0 to 70, with higher scores indicating greater cognitive impairment. Elderly normal adults may score as low as 0 or 1, but it is not unusual for non-demented adults to score slightly higher.
The patients recruited as participants in each study using the tablet formulation had mean scores on ADAS-cog of approximately 27 units, with a range from 5 to 69. Experience gained in longitudinal studies of ambulatory patients with mild to moderate Alzheimer's disease suggests that they gain 6 to 12 units a year on the ADAS-cog. Lesser degrees of change, however, are seen in patients with very mild or very advanced disease because the ADAS-cog is not uniformly sensitive to change over the course of the disease. The annualized rate of decline in the placebo patients participating in galantamine trials was approximately 4.5 units per year.
The ability of galantamine to produce an overall clinical effect was assessed using a Clinician's Interview Based Impression of Change that required the use of caregiver information, the CIBIC-plus. The CIBIC-plus is not a single instrument and is not a standardized instrument like the ADAS-cog. Clinical trials for investigational drugs have used a variety of CIBIC formats, each different in terms of depth and structure. As such, results from a CIBIC-plus reflect clinical experience from the trial or trials in which it was used and cannot be compared directly with the results of CIBIC-plus evaluations from other clinical trials. The CIBIC-plus used in the trials was a semi-structured instrument based on a comprehensive evaluation at baseline and subsequent time-points of 4 major areas of patient function: general, cognitive, behavioral and activities of daily living. It represents the assessment of a skilled clinician based on his/her observation at an interview with the patient, in combination with information supplied by a caregiver familiar with the behavior of the patient over the interval rated. The CIBIC-plus is scored as a seven-point categorical rating, ranging from a score of 1, indicating "markedly improved," to a score of 4, indicating "no change" to a score of 7, indicating "marked worsening." The CIBIC-plus has not been systematically compared directly to assessments not using information from caregivers (CIBIC) or other global methods.
14.2 Immediate-Release Tablets
U.S. Twenty-One Week Fixed-Dose Study
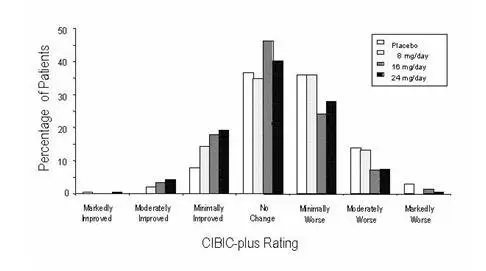
In a study of 21 weeks duration, 978 patients were randomized to doses of 8, 16, or 24 mg of galantamine per day, or to placebo, each given in 2 divided doses. Treatment was initiated at 8 mg/day for all patients randomized to galantamine and increased by 8 mg/day every 4 weeks. Therefore, the maximum titration phase was 8 weeks and the minimum maintenance phase was 13 weeks (in patients randomized to 24 mg/day of galantamine).
Effects on the ADAS-cog
Figure 1 illustrates the time course for the change from baseline in ADAS-cog scores for all four dose groups over the 21 weeks of the study. At 21 weeks of treatment, the mean differences in the ADAS-cog change scores for the galantamine-treated patients compared to the patients on placebo were 1.7, 3.3, and 3.6 units for the 8, 16 and 24 mg/day treatments, respectively. The 16 mg/day and 24 mg/day treatments were statistically significantly superior to placebo and to the 8 mg/day treatment. There was no statistically significant difference between the 16 mg/day and 24 mg/day dose groups.
Figure 1: Time-Course of the Change From Baseline in ADAS-cog Score for Patients Completing 21 Weeks (5 Months) of Treatment
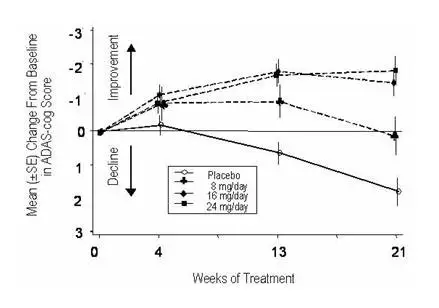
Figure 2 illustrates the cumulative percentages of patients from each of the four treatment groups who had attained at least the measure of improvement in ADAS-cog score shown on the X-axis. Three change scores (10-point, 7-point and 4-point reductions) and no change in score from baseline have been identified for illustrative purposes, and the percent of patients in each group achieving that result is shown in the inset table.
The curves demonstrate that both patients assigned to galantamine and placebo have a wide range of responses, but that the galantamine groups are more likely to show the greater improvements.
Figure 2: Cumulative Percentage of Patients Completing 21 Weeks of Double-Blind Treatment With Specified Changes From Baseline in ADAS-cog Scores. The Percentages of Randomized Patients Who Completed the Study Were: Placebo 84%, 8 mg/day 77%, 16 mg/day 78% and 24 mg/day 78%.
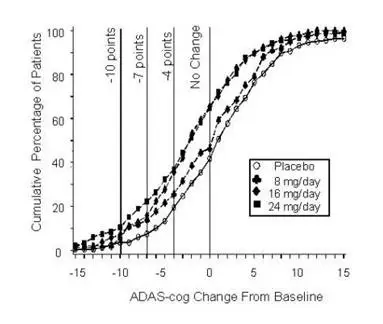
| Change in ADAS-cog | ||||
|---|---|---|---|---|
| Treatment | -10 | -7 | -4 | -0 |
| Placebo | 3.6% | 7.6% | 19.6 % | 41.8% |
| 8 mg/day | 5.9% | 13.9% | 25.7% | 46.5% |
| 16 mg/day | 7.2% | 15.9% | 35.6% | 65.4% |
| 24 mg/day | 10.4% | 22.3% | 37.0% | 64.9% |
U.S. Twenty-Six Week Fixed-Dose Study
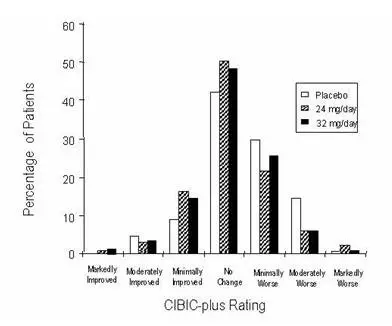
In a study of 26 weeks duration, 636 patients were randomized to either a dose of 24 mg or 32 mg of galantamine per day, or to placebo, each given in two divided doses. The 26-week study was divided into a 3-week dose titration phase and a 23-week maintenance phase.
Effects on the ADAS-cog
Figure 4 illustrates the time course for the change from baseline in ADAS-cog scores for all three dose groups over the 26 weeks of the study. At 26 weeks of treatment, the mean differences in the ADAS-cog change scores for the galantamine-treated patients compared to the patients on placebo were 3.9 and 3.8 units for the 24 mg/day and 32 mg/day treatments, respectively. Both treatments were statistically significantly superior to placebo but were not significantly different from each other.
Figure 4: Time-Course of the Change From Baseline in ADAS-cog Score for Patients Completing 26 Weeks of Treatment
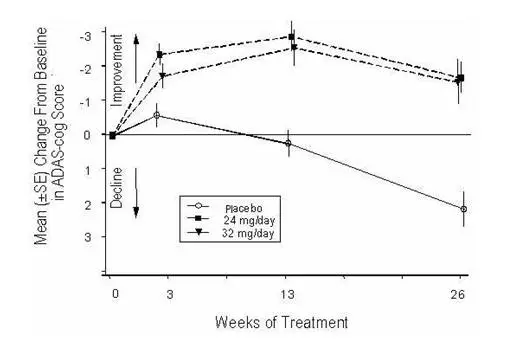
Figure 5 illustrates the cumulative percentages of patients from each of the three treatment groups who had attained at least the measure of improvement in ADAS-cog score shown on the X-axis. Three change scores (10-point, 7-point and 4-point reductions) and no change in score from baseline have been identified for illustrative purposes, and the percent of patients in each group achieving that result is shown in the inset table.
The curves demonstrate that both patients assigned to galantamine and placebo have a wide range of responses, but that the galantamine groups are more likely to show the greater improvements. A curve for an effective treatment would be shifted to the left of the curve for placebo, while an ineffective or deleterious treatment would be superimposed upon or shifted to the right of the curve for placebo, respectively.
| Change in ADAS-cog | ||||
|---|---|---|---|---|
| Treatment | -10 | -7 | -4 | -0 |
| Placebo | 2.1% | 5.7% | 16.6 % | 43.9% |
| 24 mg/day | 7.6% | 18.3% | 33.6% | 64.1% |
| 32 mg/day | 11.1% | 19.7% | 33.3% | 58.1% |
Figure 5: Cumulative Percentage of Patients Completing 26 Weeks of Double-Blind Treatment With Specified Changes From Baseline in ADAS-cog Scores. The Percentages of Randomized Patients Who Completed the Study Were: Placebo 81%, 24 mg/day 68%, and 32 mg/day 58%.
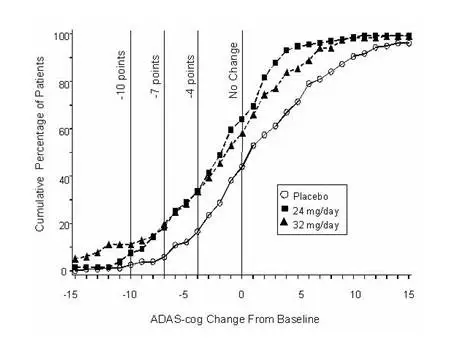
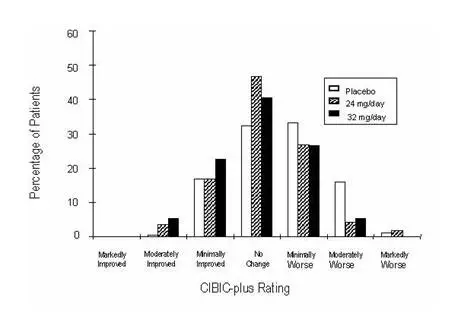
International Twenty-Six Week Fixed-Dose Study
In a study of 26 weeks duration identical in design to the USA 26-Week Fixed-Dose Study, 653 patients were randomized to either a dose of 24 mg or 32 mg of galantamine per day, or to placebo, each given in two divided doses. The 26-week study was divided into a 3-week dose titration phase and a 23-week maintenance phase.
Effects on the ADAS-cog
Figure 7 illustrates the time course for the change from baseline in ADAS-cog scores for all three dose groups over the 26 weeks of the study. At 26 weeks of treatment, the mean differences in the ADAS-cog change scores for the galantamine-treated patients compared to the patients on placebo were 3.1 and 4.1 units for the 24 mg/day and 32 mg/day treatments, respectively. Both treatments were statistically significantly superior to placebo but were not significantly different from each other.
Figure 7: Time-Course of the Change From Baseline in ADAS-cog Score for Patients Completing 26 Weeks of Treatment
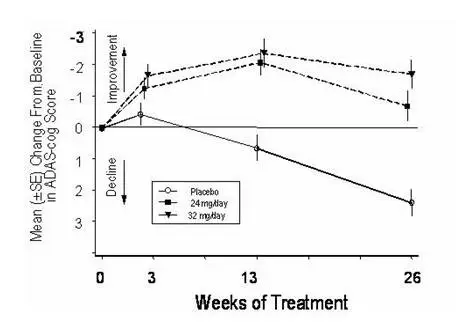
Figure 8 illustrates the cumulative percentages of patients from each of the three treatment groups who had attained at least the measure of improvement in ADAS-cog score shown on the X-axis. Three change scores (10-point, 7-point and 4-point reductions) and no change in score from baseline have been identified for illustrative purposes, and the percent of patients in each group achieving that result is shown in the inset table.
The curves demonstrate that both patients assigned to galantamine and placebo have a wide range of responses, but that the galantamine groups are more likely to show the greater improvements.
Figure 8: Cumulative Percentage of Patients Completing 26 Weeks of Double-Blind Treatment With Specified Changes From Baseline in ADAS-cog Scores. The Percentages of Randomized Patients Who Completed the Study Were: Placebo 87%, 24 mg/day 80%, and 32 mg/day 75%.
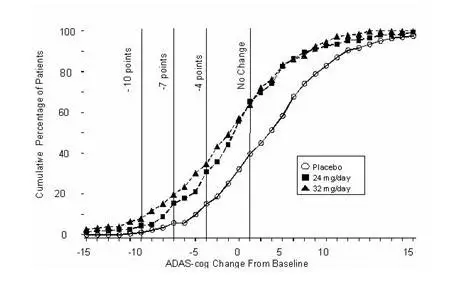
| Change in ADAS-cog | ||||
|---|---|---|---|---|
| Treatment | -10 | -7 | -4 | -0 |
| Placebo | 1.2% | 5.8% | 15.2% | 39.8% |
| 24 mg/day | 4.5% | 15.4% | 30.8% | 65.4% |
| 32 mg/day | 7.9% | 19.7% | 34.9% | 63.8% |
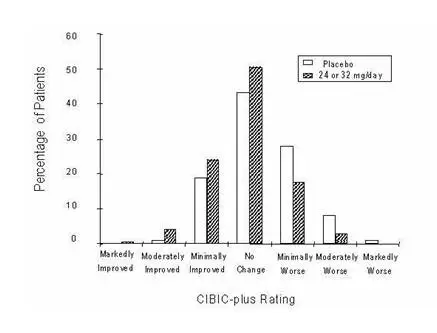
International Thirteen-Week Flexible-Dose Study
In a study of 13 weeks duration, 386 patients were randomized to either a flexible dose of 24–32 mg/day of galantamine or to placebo, each given in two divided doses. The 13-week study was divided into a 3-week dose titration phase and a 10-week maintenance phase. The patients in the active treatment arm of the study were maintained at either 24 mg/day or 32 mg/day at the discretion of the investigator.
Effects on the ADAS-cog
Figure 10 illustrates the time course for the change from baseline in ADAS-cog scores for both dose groups over the 13 weeks of the study. At 13 weeks of treatment, the mean difference in the ADAS-cog change scores for the treated patients compared to the patients on placebo was 1.9. Galantamine at a dose of 24–32 mg/day was statistically significantly superior to placebo.
Figure 10: Time-Course of the Change From Baseline in ADAS-cog Score for Patients Completing 13 Weeks of Treatment
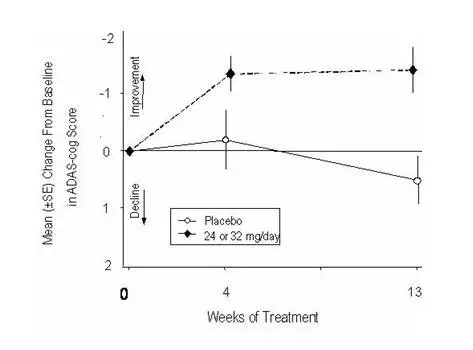
Figure 11 illustrates the cumulative percentages of patients from each of the two treatment groups who had attained at least the measure of improvement in ADAS-cog score shown on the X-axis. Three change scores (10-point, 7-point and 4-point reductions) and no change in score from baseline have been identified for illustrative purposes, and the percent of patients in each group achieving that result is shown in the inset table.
The curves demonstrate that both patients assigned to galantamine and placebo have a wide range of responses, but that the galantamine group is more likely to show the greater improvement.
Figure 11: Cumulative Percentage of Patients Completing 13 Weeks of Double-Blind Treatment With Specified Changes from Baseline in ADAS-cog Scores. The Percentages of Randomized Patients Who Completed the Study Were: Placebo 90%, 24–32 mg/day 67%.
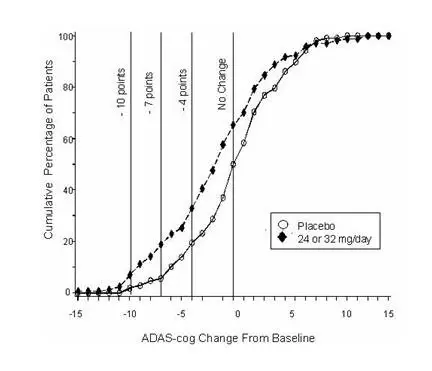
| Change in ADAS-cog | ||||
|---|---|---|---|---|
| Treatment | -10 | -7 | -4 | -0 |
| Placebo | 1.9% | 5.6% | 19.4% | 50.0% |
| 24 or 32 mg/day | 7.1% | 18.8% | 32.9% | 65.3% |
14.3 Extended-Release Capsules
The efficacy of galantamine extended-release capsules was studied in a randomized, double-blind, placebo-controlled trial which was 6 months in duration and had an initial 4-week dose-escalation phase. In this trial, patients were assigned to one of 3 treatment groups: Galantamine extended-release capsules in a flexible dose of 16 to 24 mg once daily; galantamine tablets in a flexible dose of 8 to 12 mg twice daily; and placebo. The primary efficacy measures in this study were the ADAS-cog and CIBIC-plus. On the protocol-specified primary efficacy analysis at Month 6, a statistically significant improvement favoring galantamine extended-release capsules over placebo was seen for the ADAS-cog, but not for the CIBIC-plus. Galantamine extended-release capsules showed a statistically significant improvement when compared with placebo on the Alzheimer's Disease Cooperative Study-Activities of Daily Living (ADCS-ADL) scale, a measure of function, and a secondary efficacy measure in this study. The effects of both galantamine extended-release capsules and galantamine tablets on the ADAS-cog, CIBIC-plus, and ADCS-ADL were similar in this study.
| RAZADYNE
galantamine hydrobromide capsule, extended release |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| RAZADYNE
galantamine hydrobromide capsule, extended release |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| RAZADYNE
galantamine hydrobromide capsule, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| RAZADYNE
galantamine hydrobromide tablet, film coated |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| RAZADYNE
galantamine hydrobromide tablet, film coated |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| RAZADYNE
galantamine hydrobromide tablet, film coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Janssen Pharmaceuticals, Inc. (063137772) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Ortho LLC | 805887986 | MANUFACTURE(50458-387, 50458-388, 50458-389, 50458-396, 50458-397, 50458-398) , PACK(50458-387, 50458-388, 50458-389, 50458-396, 50458-397, 50458-398) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Pharmaceutica NV | 400345889 | API MANUFACTURE(50458-387, 50458-388, 50458-389, 50458-396, 50458-397, 50458-398) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Pharmaceuticals, Inc | 063137772 | ANALYSIS(50458-387, 50458-388, 50458-389, 50458-396, 50458-397, 50458-398) | |