Drug Detail:Voxzogo (Vosoritide)
Drug Class: Miscellaneous hormones
Highlights of Prescribing Information
VOXZOGO (vosoritide) for injection, for subcutaneous use
Initial U.S. Approval: 2021
Indications and Usage for Voxzogo Injection
VOXZOGO is a C type natriuretic peptide (CNP) analog indicated to increase linear growth in pediatric patients with achondroplasia who are 5 years of age and older with open epiphyses. This indication is approved under accelerated approval based on an improvement in annualized growth velocity. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s). (1)
Voxzogo Injection Dosage and Administration
- Ensure adequate food and fluid intake prior to administration. (2.1)
- Recommended dosage is based on patient's weight. Administer subcutaneously once daily. (2.2)
- Reconstitute prior to use. The injection volume is based on both patient's weight and concentration of reconstituted VOXZOGO. (2.2)
- Monitor growth and adjust dosage according to body weight. Permanently discontinue upon closure of epiphyses. (2.3)
- See full prescribing information for preparation and administration instructions. (2.4)
Dosage Forms and Strengths
For injection: 0.4 mg, 0.56 mg, or 1.2 mg lyophilized powder in a single-dose vial for reconstitution. (3)
Contraindications
None. (4)
Warnings and Precautions
Risk of Low Blood Pressure: Transient decreases in blood pressure have been reported. (5.1)
Adverse Reactions/Side Effects
Most common adverse reactions (>10%) are injection site erythema, injection site swelling, vomiting, injection site urticaria, arthralgia, decreased blood pressure, and gastroenteritis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact BioMarin Pharmaceutical Inc. at 1-866-906-6100, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Renal Impairment: Not recommended in patients with eGFR < 60 mL/min/1.73 m2. (8.6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2021
Full Prescribing Information
1. Indications and Usage for Voxzogo Injection
VOXZOGO is indicated to increase linear growth in pediatric patients with achondroplasia who are 5 years of age and older with open epiphyses. This indication is approved under accelerated approval based on an improvement in annualized growth velocity [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s).
2. Voxzogo Injection Dosage and Administration
2.1 Important Instructions Prior to Administration of VOXZOGO
To reduce the risk of low blood pressure and its associated signs and symptoms, instruct the caregiver and patient that the patient should [see Warnings and Precautions (5.1)]:
- Have adequate food intake prior to VOXZOGO administration.
- Drink approximately 240-300 mL of fluid in the hour prior to VOXZOGO administration.
2.2 Recommended Dosage and Administration
The recommended dosage of VOXZOGO is based on the patient's actual body weight (see Table 1). VOXZOGO is administered by subcutaneous injection once daily [see Dosage and Administration (2.4)].
Inject VOXZOGO at approximately the same time each day, if possible. The volume of VOXZOGO to be administered (injection volume) is based on the patient's actual body weight and the concentration of reconstituted VOXZOGO (0.8 mg/mL or 2 mg/mL) (Table 1). VOXZOGO must be reconstituted prior to use [see Dosage and Administration (2.4)].
| Actual Body Weight | Vial Strength for Reconstitution* | Dose | Injection Volume |
|---|---|---|---|
|
|||
| 10-11 kg | 0.4 mg | 0.24 mg | 0.3 mL |
| 12-16 kg | 0.56 mg | 0.28 mg | 0.35 mL |
| 17-21 kg | 0.56 mg | 0.32 mg | 0.4 mL |
| 22-32 kg | 0.56 mg | 0.4 mg | 0.5 mL |
| 33-43 kg | 1.2 mg | 0.5 mg | 0.25 mL |
| 44-59 kg | 1.2 mg | 0.6 mg | 0.3 mL |
| 60-89 kg | 1.2 mg | 0.7 mg | 0.35 mL |
| ≥90 kg | 1.2 mg | 0.8 mg | 0.4 mL |
2.3 Growth Monitoring
Monitor and assess patient body weight, growth, and physical development regularly every 3-6 months. Adjust the dosage according to the patient's actual body weight [see Dosage and Administration (2.2)].
Permanently discontinue VOXZOGO upon confirmation of no further growth potential, indicated by closure of epiphyses.
2.4 Preparation and Administration
Reconstitute VOXZOGO before administration using the provided diluent syringe containing Sterile Water for Injection, USP (see Reconstitution Instructions below).
Caregivers may inject VOXZOGO subcutaneously after proper training by a healthcare professional on the preparation and administration of VOXZOGO [see Instructions for Use].
Reconstitution Instructions
- Select the correct VOXZOGO strength and prefilled diluent syringe co-pack based on the patient's actual body weight [see Dosage and Administration (2.2)].
- Remove VOXZOGO vial and prefilled diluent syringe (Sterile Water for Injection, USP) from the refrigerator and allow the vial and prefilled diluent syringe to reach room temperature before reconstituting VOXZOGO.
- Attach the diluent needle provided with ancillary supplies to the diluent prefilled syringe.
- Inject the entire diluent prefilled syringe volume into the vial.
- Gently swirl the diluent in the vial until the white powder is completely dissolved. Do not shake.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Once reconstituted VOXZOGO is a clear, colorless to yellow liquid. The solution should not be used if discolored or cloudy, or if particles are present. The concentration of reconstituted solution is 0.8 mg/mL or 2.0 mg/mL.
- After reconstitution, VOXZOGO can be held in the vial at a room temperature 20°C to 25°C (68°F to 77°F) for a maximum of 3 hours.
- For administration, extract the required dose volume from the vial using the supplied administration syringe [see Dosage and Administration (2.2)].
Discard any unused portion. Do not pool unused portions from the vials. Do not administer more than 1 dose from a vial. Do not mix with other medications.
3. Dosage Forms and Strengths
For Injection: 0.4 mg, 0.56 mg, or 1.2 mg as a white to yellow lyophilized powder for reconstitution in a single-dose vial.
5. Warnings and Precautions
5.1 Risk of Low Blood Pressure
Transient decreases in blood pressure were observed in clinical studies of VOXZOGO. Subjects with significant cardiac or vascular disease and patients on anti-hypertensive medicinal products were excluded from participation in VOXZOGO clinical trials. To reduce the risk of a decrease in blood pressure and associated symptoms (dizziness, fatigue and/or nausea), instruct patients to be well hydrated and have adequate food intake prior to administration of VOXZOGO [see Dosage and Administration (2.1) and Adverse Reactions (6.1)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Risk of Low Blood Pressure [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
VOXZOGO was studied in a 52-week, randomized, double-blind, placebo-controlled trial in 121 subjects with achondroplasia (Study 1) [see Clinical Studies (14)].
The subjects' ages ranged from 5.1 to 14.9 years with a mean of 8.7 years. Sixty four (53%) subjects were male and 57 (47%) were female. Overall, 86 (71%) subjects were White, 23 (19%) were Asian, 5 (4%) were Black or African American, and 7 (6%) were classified as "multiple" race. The demographic and baseline characteristics were balanced between treatment groups. The subjects received either VOXZOGO 15 mcg/kg, or placebo administered subcutaneously once daily.
Table 2 shows adverse reactions that occurred in ≥5% of patients treated with VOXZOGO and at a percentage greater than placebo.
| Adverse Reaction | Placebo (N=61) n (%) | VOXZOGO (N=60) n (%) |
|---|---|---|
| Abreviations: N, total number of subjects in the treatment arm; n, number of subjects with the adverse reaction; %, percent of subjects with the adverse reaction. | ||
|
||
| Injection site erythema | 42 (69%) | 45 (75%) |
| Injection site swelling | 22 (36%) | 37 (62%) |
| Vomiting | 12 (20%) | 16 (27%) |
| Injection site urticaria | 6 (10%) | 15 (25%) |
| Arthralgia | 4 (7%) | 9 (15%) |
| Decreased blood pressure | 3 (5%) | 8 (13%) |
| Gastroenteritis† | 5 (8%) | 8 (13%) |
| Diarrhea | 2 (3%) | 6 (10%) |
| Dizziness‡ | 2 (3%) | 6 (10%) |
| Ear pain | 3 (5%) | 6 (10%) |
| Influenza | 3 (5%) | 6 (10%) |
| Fatigue§ | 2 (3%) | 5 (8%) |
| Seasonal allergy | 1 (2%) | 4 (7%) |
| Dry skin | 0 | 3 (5%) |
6.2 Immunogenicity
As with all peptides, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
Of 131 subjects who were treated with VOXZOGO 15 mcg/kg/day and evaluable for the presence of anti-drug antibodies (ADA) for up to 240 weeks, ADA were detected in 35% (46/131). The earliest time to ADA development was day 85. All ADA-positive subjects tested negative for anti-vosoritide neutralizing antibodies. There was no correlation between the number, duration, or severity of hypersensitivity adverse reactions or injection site reactions and ADA positivity or mean ADA titer. There was no association between ADA positivity or mean ADA titer and change from baseline in annual growth velocity (AGV) or height Z-score at month 12. There was no impact of serum ADA detected on the plasma PK measurements of vosoritide.
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of VOXZOGO have been established in pediatric patients aged 5 years and older for the improvement in linear growth in patients with achondroplasia. Use of VOXZOGO for this indication is supported by evidence from adequate and well-controlled studies in pediatric patients aged 5 years and older [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)].
Safety and effectiveness of VOXZOGO in pediatric patients with achondroplasia below the age of 5 years have not been established.
11. Voxzogo Injection Description
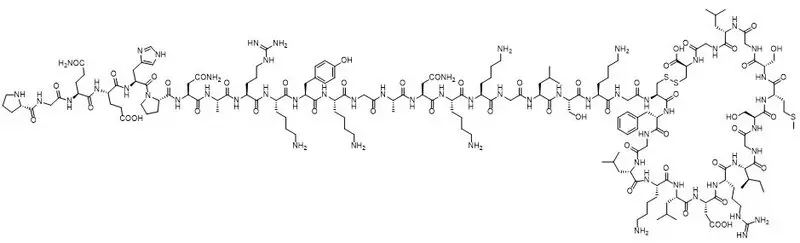
VOXZOGO contains vosoritide, a human C type natriuretic peptide (CNP) analog. Vosoritide is a 39 amino acid peptide. Its amino acid sequence includes the 37 C terminal amino acids of the human CNP53 sequence plus Pro Gly on the N terminus to convey resistance to neutral endopeptidase (NEP) degradation. Vosoritide is manufactured from Escherichia coli using recombinant DNA technology. Vosoritide has a chemical formula of C176H290N56O51S3 with a molecular weight of 4.1 kDa.
Vosoritide has the structural formula shown in Figure 1.
Figure 1
VOXZOGO (vosoritide) for injection, is a sterile, preservative-free white-to-yellow lyophilized powder, for subcutaneous administration after reconstitution with Sterile Water for Injection, USP.
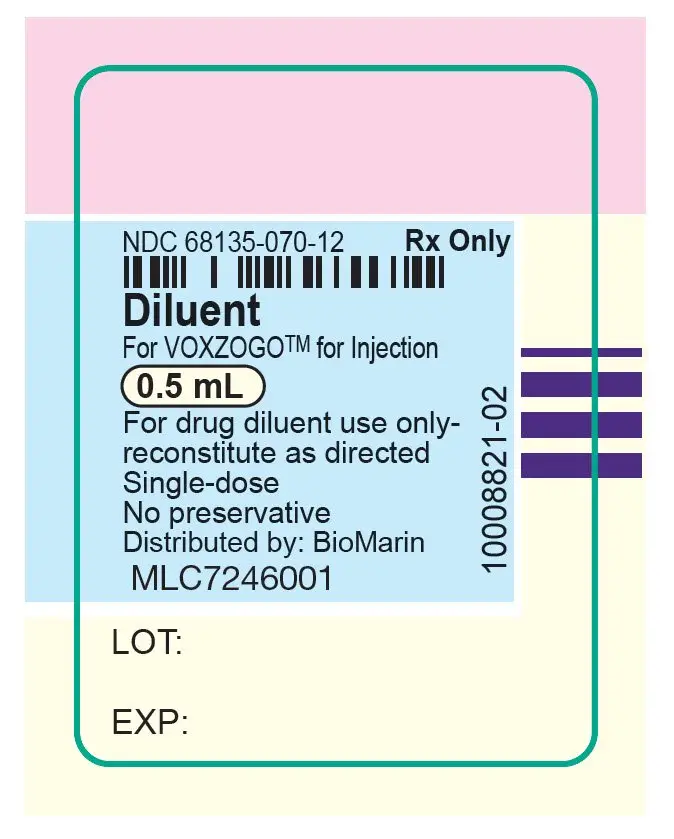
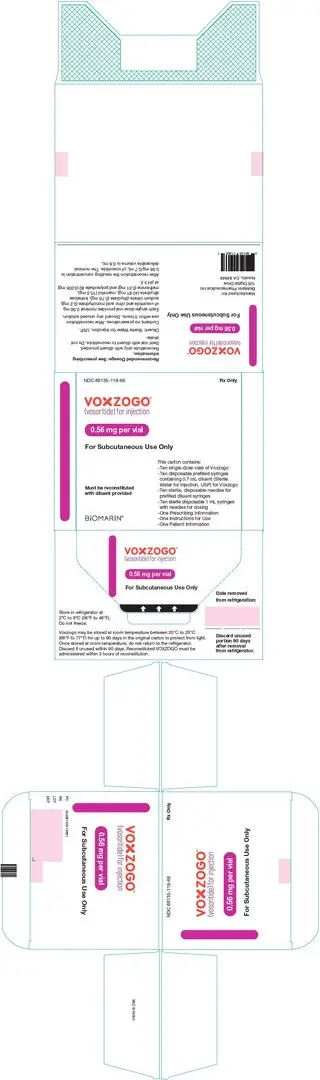
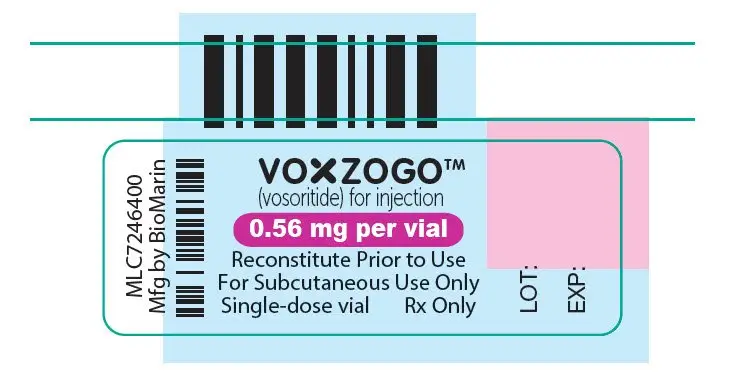
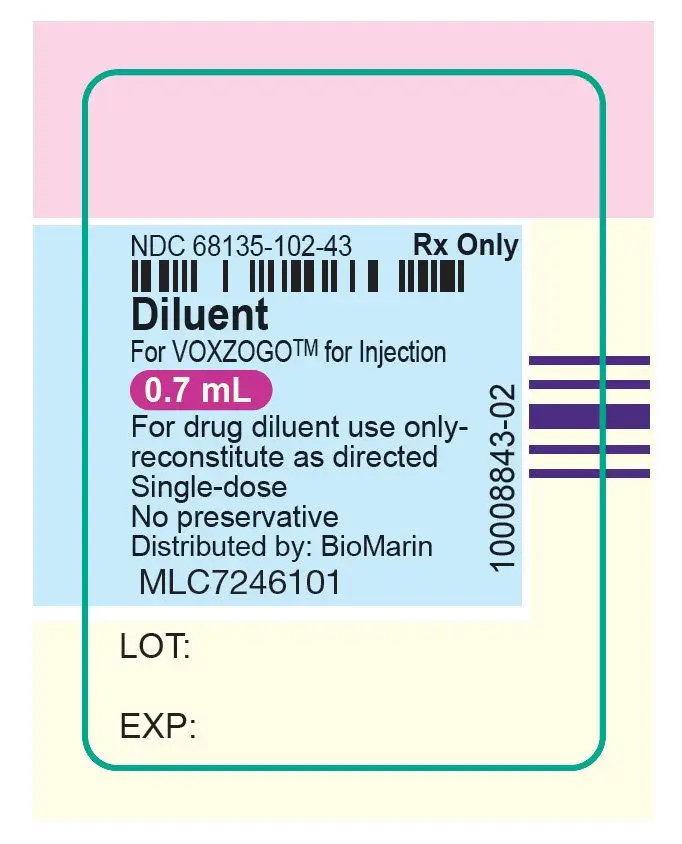
VOXZOGO is provided as a single-dose vial containing 0.4 mg, 0.56 mg, or 1.2 mg of vosoritide per vial. A pre-filled syringe containing Sterile Water for Injection, USP for use as a diluent is also provided. The contents of each single dose vial are summarized by strength in Table 3. The product contains no preservative.
| Strength | Inactive Ingredients per Vial |
|---|---|
| Trehalose dihydrate and D-Mannitol are used as isotonic agent. Citric acid monohydrate and sodium citrate dihydrate are used as buffering agent. | |
| VOXZOGO 0.4 mg | Trehalose dihydrate (29.01 mg), mannitol (7.5 mg), sodium citrate dihydrate (0.54 mg), methionine (0.36 mg), citric acid monohydrate (0.14 mg), and polysorbate 80 (0.025 mg). After reconstitution with 0.5 mL Sterile Water for Injection USP, the resulting concentration is 0.4 mg/0.5 mL of vosoritide and the nominal deliverable volume is 0.4 mL. |
| VOXZOGO 0.56 mg | Trehalose dihydrate (40.61 mg), mannitol (10.50 mg), sodium citrate dihydrate (0.76 mg), methionine (0.51 mg), citric acid monohydrate (0.20 mg), and polysorbate 80 (0.035 mg). After reconstitution with 0.7 mL Sterile Water for Injection USP, the resulting concentration is 0.56 mg/0.7 mL of vosoritide and the nominal deliverable volume is 0.6 mL. |
| VOXZOGO 1.2 mg | Trehalose dihydrate (34.81 mg), mannitol (9 mg), sodium citrate dihydrate (0.65 mg), methionine (0.44 mg), citric acid monohydrate (0.17 mg), and polysorbate 80 (0.030 mg). After reconstitution with 0.6 mL Sterile Water for Injection USP, the resulting concentration is 1.2 mg/0.6 mL of vosoritide and the nominal deliverable volume is 0.5 mL. |
12. Voxzogo Injection - Clinical Pharmacology
12.1 Mechanism of Action
In patients with achondroplasia, endochondral bone growth is negatively regulated due to a gain of function mutation in fibroblast growth factor receptor 3 (FGFR3). Binding of vosoritide to natriuretic peptide receptor-B (NPR-B) antagonizes FGFR3 downstream signaling by inhibiting the extracellular signal-regulated kinases 1 and 2 (ERK1/2) in the mitogen-activated protein kinase (MAPK) pathway at the level of rapidly accelerating fibrosarcoma serine/threonine protein kinase (RAF-1). As a result, vosoritide, like CNP, acts as a positive regulator of endochondral bone growth as it promotes chondrocyte proliferation and differentiation.
In animal models with open growth plates, vosoritide administration resulted in the promotion of chondrocyte proliferation and differentiation that led to a widening of the growth plate and subsequent increase in skeletal growth. In the mouse models of FGFR3-related chondrodysplasia, a partial or complete normalization of the dwarfism phenotype was observed.
12.3 Pharmacokinetics
The area under the concentration-time curve (AUC) and peak concentration (Cmax) of vosoritide increased greater than proportionally following subcutaneous administration to pediatric subjects with achondroplasia in the dose range of 7.5 to 30.0 mcg/kg. The pharmacokinetics of vosoritide were evaluated in 58 subjects aged 5 to 13 years with achondroplasia who received subcutaneous injections of vosoritide 15 mcg/kg once daily for 52 weeks. The mean (± SD) Cmax and area under the concentration-time curve from time zero to the last measurable concentration (AUC0-t) observed across 52 weeks of treatment ranged from 4.71 (± 2.32) to 7.18 (± 9.65) ng/mL, and 161 (± 98.1) to 290 (± 235) ng-min/mL, respectively. No drug accumulation was observed following 15 mcg/kg once daily dosing. The exposure of vosoritide increased with the duration of treatment. The mean AUC0-t at week 52 increased approximately 20% compared to that at day 1.
13. Nonclinical Toxicology
14. Clinical Studies
The safety and effectiveness of VOXZOGO in patients with achondroplasia were assessed in one 52-week, multi-center, randomized, double-blind, placebo-controlled, phase 3 study - Study 1 (NCT03197766).
Study 1 was conducted in 121 subjects with genetically-confirmed achondroplasia, who were randomized to either VOXZOGO (N=60) or placebo (N=61). The dosage of VOXZOGO was 15 mcg/kg administered subcutaneously once daily. Baseline standing height, weight Z-score, body mass index (BMI) Z-score, and upper to lower body ratio were collected for at least 6 months prior to randomization. Subjects with limb-lengthening surgery in the prior 18 months or who planned to have limb-lengthening surgery during the study period were excluded. The study included a 52-week placebo-controlled treatment phase followed by an open-label treatment extension study period in which all subjects received VOXZOGO. The primary efficacy endpoint was the change from baseline in annualized growth velocity (AGV) at Week 52 compared with placebo.
The subjects' ages ranged from 5.1 to 14.9 years with a mean of 8.7 years. Sixty four (53%) subjects were male and 57 (47%) were female. Overall, 86 (71%) subjects were White, 23 (19%) were Asian, 5 (4%) were Black or African American, and 7 (6%) were classified as "multiple" race. The subjects had a mean baseline height standard deviation score (SDS) of -5.13.
Treatment with VOXZOGO for 52 weeks resulted in a treatment difference in the change from baseline in AGV of 1.57 cm/year after 52 weeks of treatment (Table 4).
| Placebo (N=61*) | VOXZOGO 15 mcg/kg Daily (N=60*) |
|
|---|---|---|
| Abbreviations: AGV, annualized growth velocity; 95% CI, 95% confidence interval; LS, least-square; SD, standard deviation | ||
|
||
| Baseline mean (SD)† | 4.06 (1.20) | 4.26 (1.53) |
| Change from baseline‡ | -0.17 | 1.40 |
| Difference in change of VOXZOGO – Placebo‡
(95% CI) | 1.57 (1.22, 1.93)§ |
|
The improvement in AGV in favor of VOXZOGO was consistent across all predefined subgroups analyzed including sex, age group, Tanner stage, baseline height Z-score, and baseline AGV.
17. Patient Counseling Information
Advise the patient and caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
| PATIENT INFORMATION VOXZOGO (vox zoeʹ goe) (vosoritide) for injection, for subcutaneous use |
|
|---|---|
| This Patient Information has been approved by the U.S. Food and Drug Administration | Issued: 11/2021 |
| What is VOXZOGO?
VOXZOGO is a prescription medicine used to increase linear growth in children with achondroplasia who are 5 years of age and older with open bone growth plates (epiphyses). It is not known if VOXZOGO is safe and effective in children with achondroplasia under 5 years of age. |
|
Before you give your child VOXZOGO, tell your child's healthcare provider about all your child's medical conditions, including if they:
Know the medicines your child takes. Keep a list of them to show your child's healthcare provider and pharmacist when your child gets a new medicine. |
|
How should I give VOXZOGO?
|
|
| What are the possible side effects of VOXZOGO? VOXZOGO may cause serious side effects, including:
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store VOXZOGO?
|
|
| General information about the safe and effective use of VOXZOGO.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use VOXZOGO for a condition for which it was not prescribed. Do not give VOXZOGO to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about VOXZOGO that is written for health professionals. |
|
| What are the ingredients in VOXZOGO?
Active ingredient: vosoritide Inactive ingredients: trehalose dihydrate, mannitol, sodium citrate dihydrate, methionine, citric acid monohydrate, and polysorbate 80 BioMarin Pharmaceutical Inc. Novato, CA 94949 © BioMarin Pharmaceutical Inc. All rights reserved. VOXZOGO is a registered trademark of BioMarin Pharmaceutical Inc. For more information, go to www.VOXZOGO.com or call 1-877-695-8826. |
|
INSTRUCTIONS FOR USEVOXZOGO™ [Vox zoeʹ goe](vosoritide)for injection, for subcutaneous useSingle-Use
This Instructions for Use contains information for caregivers on how to inject VOXZOGO.
Read this Instructions for Use before you start using VOXZOGO and each time you get a refill. There may be new information. This information does not take the place of talking to your child's healthcare provider about your child's medical condition and their treatment. Before you use VOXZOGO for the first time, make sure your child's healthcare provider shows you the right way to use it. Contact your child's healthcare provider if you or your child have any questions.
Important Information You Need to Know Before Injecting VOXZOGO
- Wash your hands with soap and water.
- Do not drop VOXZOGO or put opened items down on surfaces that are not clean.
- VOXZOGO is available in more than 1 strength. Make sure the strength matches your prescription strength. Do not open packaging until ready to use.
- Take the VOXZOGO vial and prefilled diluent syringe out of the refrigerator and allow them to reach room temperature before mixing.
- Inspect the vial and supplies for any signs of damage or contamination. Do not use if damaged or contaminated.
- Check the expiration date. The expiration date can be found on the carton, vial and prefilled diluent syringe. Do not use if expired.
- Your child should eat a meal and drink a glass (about 8 to 10 ounces) of fluid (such as water, milk, or juice) within 1 hour before injection.
- VOXZOGO should be given at about the same time every day.
- Do not mix VOXZOGO with other medicines.
- After mixing the VOXZOGO, use it right away. Do not use the mixed VOXZOGO if it has been sitting at room temperature for more than 3 hours. Throw it away (dispose of) in a sharps container. See step 18 and "How to Throw Away (Dispose of) VOXZOGO" for more information.
- Do not reuse any of the supplies. After the injection, throw away (dispose of) the used vial even if there is VOXZOGO remaining. See step 18 and "How to Throw Away (Dispose of) VOXZOGO" for more information.
How to Store VOXZOGO
- Store the VOXZOGO vial and prefilled diluent syringe in the refrigerator between 36°F to 46°F (2°C to 8°C).
- You may store VOXZOGO (before mixing) at room temperature between 68°F to 77°F (20°C to 25°C) for 90 days. Record the date you started storing VOXZOGO at room temperature on the carton to keep track of the 90 days. Do not return VOXZOGO to the refrigerator after it has been stored at room temperature. Throw VOXZOGO away if unused within 90 days of storing at room temperature.
- Do not freeze VOXZOGO.
- Store VOXZOGO out of direct sunlight.
Keep VOXZOGO and all other medicines out of the reach of children.
Supplies Needed to Inject VOXZOGO
Gather all of these supplies on a clean, flat surface before injecting.
| Items supplied | Items not supplied
If you do not have these items, ask your pharmacist. |
|
VOXZOGO
 |  |
|
 |  |
|
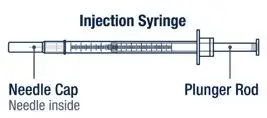
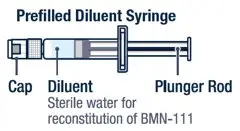 |  |
|
 |
Preparing VOXZOGO for Injection
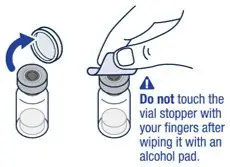
| ▶ Step 1
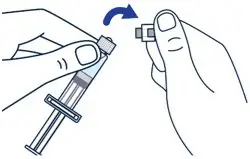
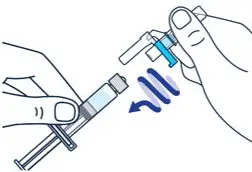
On a clean flat surface, flip off the vial cap and wipe the top with an alcohol pad. | ▶ Step 2
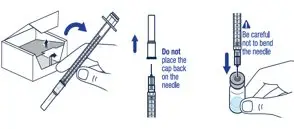
Gently bend to snap off the cap from the prefilled diluent syringe. | ▶ Step 3
Twist the diluent needle onto the prefilled diluent syringe until you can no longer twist it. Do not use the prefilled diluent syringe to give the injection. |
||
 |  |  |
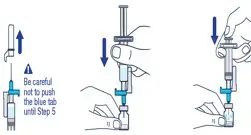
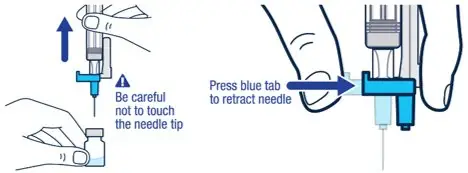
| ▶ Step 4
Pull off the needle cap and insert the needle through the middle of the vial stopper. Slowly push the plunger rod down to inject all of the liquid. | ▶ Step 5
Remove the needle from the vial, then press the blue tab for the needle to pull back (retract). Throw away the needle and syringe in a sharps container. See step 18 and "How to Throw Away (Dispose of) VOXZOGO." Do not use the prefilled diluent syringe to give the injection. |
|||
 |  |
|||
| ▶ Step 6
Gently swirl the vial until the powder has completely dissolved and the solution is clear. Do not shake. | ▶ Step 7
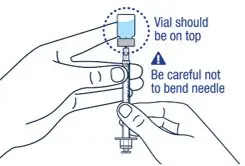
Take the injection syringe out of the carton. Pull off the needle cap from the injection syringe and insert the needle straight through the middle of the vial stopper. Be careful not to bend the needle. | ▶ Step 8
Carefully hold the vial and syringe and turn the vial upside down with the needle still inserted. The vial should be on top. Be careful not to bend the needle. |
||
 |  |  |
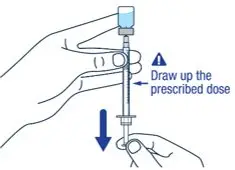
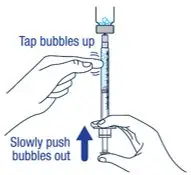
| ▶ Step 9
Keep the needle tip in the medicine and slowly pull the plunger rod back to draw up the prescribed dose in the syringe. Check the prescription label for how much to draw up. | ▶ Step 10
Remove large air bubbles in the syringe by gently tapping the syringe. Then push the bubbles back into the vial. | |||
 |  |
| ▶ Step 11
Repeat steps 9 and 10 until you have the correct prescribed dose in the syringe and no large bubbles. | ▶ Step 12
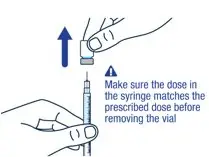
Make sure you have the prescribed dose in the syringe, then remove the vial and prepare to give the dose. |
|||
 |  |
|||
Select and Prepare Injection Site
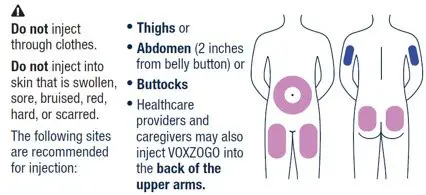
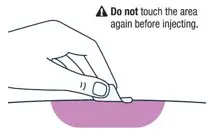
| ▶ Step 13 | ▶ Step 14 | |||
| VOXZOGO should be injected into the fatty layer under the skin (subcutaneous) only. Do not inject into the same site 2 times in a row. | Wipe the injection site with an alcohol pad and let the skin air dry. | |||
 |  |
|||
Giving VOXZOGO Injection
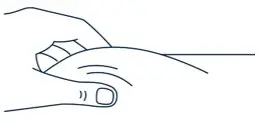
| ▶ Step 15 | ▶ Step 16 | |||
| After wiping the site with an alcohol pad, pinch the skin up around the selected injection site. | Quickly insert the needle all the way into the skin at a 45-degree angle. | |||
 | 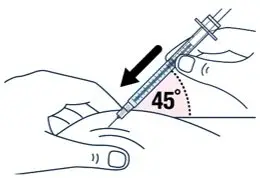 |
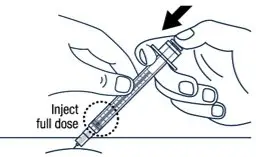
| ▶ Step 17 Release the pinch and slowly push the plunger rod all the way down. |
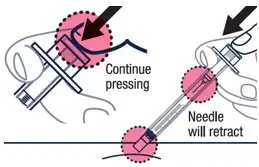
 | Continue pressing the plunger rod until the needle retracts into the syringe. | ▶ Step 18 Throw away the used vial, syringes and needles in a sharps container. See "How to Throw Away (Dispose of) VOXZOGO" for more information. |
|
 |  | 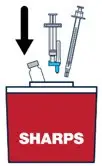 |
How to Throw Away (Dispose of) VOXZOGO
Put your used or expired vials, needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the vials, loose needles and syringes in your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that:
- is made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid without sharps being able to come out,
- is upright and stable during use,
- is leak-resistant, and
- is properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be local or state laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
After the Injection
- Inspect the injection site. If a small amount of bleeding occurs from the injection site, gently press a gauze pad on it for a few seconds or apply a bandage. Do not rub the injection site.
- Monitor for signs of low blood pressure, such as dizziness, tiredness, and nausea. If your child experiences these symptoms you should call your child's healthcare provider, then get your child to lay back with legs raised.
For Help or More Information
- Call your healthcare provider
- Call BioMarin at 1-800-123-4567
- Visit www.VOXZOGO.com
Manufactured for:
BioMarin Pharmaceutical Inc.,
Novato, CA 94949
REP-5233-C10
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Approved: November 2021
| VOXZOGO 0.4MG
vosoritide kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| VOXZOGO 0.56MG
vosoritide kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| VOXZOGO 1.2MG
vosoritide kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - BioMarin Pharmaceutical Inc. (079722386) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vetter Pharma-Fertigung Gmbh & Co. KG | 312670654 | ANALYSIS(68135-082, 68135-119, 68135-181) , MANUFACTURE(68135-082, 68135-119, 68135-181) , PACK(68135-082, 68135-119, 68135-181) | |