Drug Detail:Invega trinza (Paliperidone palminate)
Generic Name: paliperidone palmitate 273mg in 0.88mL
Dosage Form: injection, suspension, extended release
Drug Class: Atypical antipsychotics
Administration Instructions
INVEGA TRINZA® should be administered once every 3 months.
Each injection must be administered only by a healthcare professional.
Parenteral drug products should be inspected visually for foreign matter and discoloration prior to administration. It is important to shake the syringe vigorously for at least 15 seconds to ensure a homogeneous suspension. Inject INVEGA TRINZA® within 5 minutes of shaking vigorously [see Dosage and Administration (2.8)].
INVEGA TRINZA® is intended for intramuscular use only. Do not administer by any other route. Avoid inadvertent injection into a blood vessel. Administer the dose in a single injection; do not administer the dose in divided injections. Inject slowly, deep into the deltoid or gluteal muscle.
INVEGA TRINZA® must be administered using only the thin wall needles that are provided in the INVEGA TRINZA® pack. Do not use needles from the 1-month paliperidone palmitate extended-release injectable suspension pack or other commercially-available needles to reduce the risk of blockage.
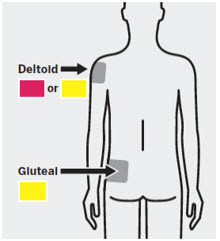
Deltoid Injection
The recommended needle size for administration of INVEGA TRINZA® into the deltoid muscle is determined by the patient's weight:
- For patients weighing less than 90 kg, the 1-inch, 22 gauge thin wall needle is recommended.
- For patients weighing 90 kg or more, the 1½-inch, 22 gauge thin wall needle is recommended.
Administer into the center of the deltoid muscle. Deltoid injections should be alternated between the two deltoid muscles.
Gluteal Injection
Regardless of patient weight, the recommended needle size for administration of INVEGA TRINZA® into the gluteal muscle is the 1½-inch, 22 gauge thin wall needle. Administer into the upper-outer quadrant of the gluteal muscle. Gluteal injections should be alternated between the two gluteal muscles.
Incomplete Administration
To avoid an incomplete administration of INVEGA TRINZA®, ensure that the prefilled syringe is shaken vigorously for at least 15 seconds within 5 minutes prior to administration to ensure a homogeneous suspension and ensure the needle does not get clogged during injection [see Dosage and Administration (2.8)].
However, in the event of an incompletely administered dose, do not re-inject the dose remaining in the syringe and do not administer another dose of INVEGA TRINZA®. Closely monitor and treat the patient with oral supplementation as clinically appropriate until the next scheduled 3-month injection of INVEGA TRINZA®.
Schizophrenia
Adults
INVEGA TRINZA® is to be used only after INVEGA SUSTENNA® (1-month paliperidone palmitate extended-release injectable suspension) has been established as adequate treatment for at least four months. In order to establish a consistent maintenance dose, it is recommended that the last two doses of INVEGA SUSTENNA® be the same dosage strength before starting INVEGA TRINZA®.
Initiate INVEGA TRINZA® when the next 1-month paliperidone palmitate dose is scheduled with an INVEGA TRINZA® dose based on the previous 1-month injection dose, using the equivalent 3.5-fold higher dose as shown in Table 1. INVEGA TRINZA® may be administered up to 7 days before or after the monthly time point of the next scheduled paliperidone palmitate 1-month dose.
| If the Last Dose of INVEGA SUSTENNA® is: | Initiate INVEGA TRINZA® at the Following Dose: |
|---|---|
| Conversion from the INVEGA SUSTENNA® 39 mg dose was not studied. | |
| 78 mg | 273 mg |
| 117 mg | 410 mg |
| 156 mg | 546 mg |
| 234 mg | 819 mg |
Following the initial INVEGA TRINZA® dose, INVEGA TRINZA® should be administered every 3 months. If needed, dose adjustment can be made every 3 months in increments within the range of 273 mg to 819 mg based on individual patient tolerability and/or efficacy. Due to the long-acting nature of INVEGA TRINZA®, the patient's response to an adjusted dose may not be apparent for several months [see Clinical Pharmacology (12.3)].
Missed Doses
Dosing Window
Missing doses of INVEGA TRINZA® should be avoided. If necessary, patients may be given the injection up to 2 weeks before or after the 3-month time point.
Missed Dose 3½ Months to 4 Months Since Last Injection
If more than 3½ months (up to but less than 4 months) have elapsed since the last injection of INVEGA TRINZA®, the previously administered INVEGA TRINZA® dose should be administered as soon as possible, then continue with the 3-month injections following this dose.
Missed Dose 4 Months to 9 Months Since Last Injection
If 4 months up to and including 9 months have elapsed since the last injection of INVEGA TRINZA®, do NOT administer the next dose of INVEGA TRINZA®. Instead, use the re-initiation regimen shown in Table 2.
| If the Last Dose of INVEGA TRINZA® was: | Administer INVEGA SUSTENNA®, two doses one week apart (into deltoid muscle) | Then administer INVEGA TRINZA® (into deltoid* or gluteal muscle) | |
|---|---|---|---|
| Day 1 | Day 8 | 1 month after Day 8 | |
|
|||
| 273 mg | 78 mg | 78 mg | 273 mg |
| 410 mg | 117 mg | 117 mg | 410 mg |
| 546 mg | 156 mg | 156 mg | 546 mg |
| 819 mg | 156 mg | 156 mg | 819 mg |
Missed Dose Longer than 9 Months Since Last Injection
If more than 9 months have elapsed since the last injection of INVEGA TRINZA®, re-initiate treatment with the 1-month paliperidone palmitate extended-release injectable suspension as described in the prescribing information for that product. INVEGA TRINZA® can then be resumed after the patient has been adequately treated with the 1-month paliperidone palmitate extended-release injectable suspension for at least 4 months.
Use with Risperidone or with Oral Paliperidone
Since paliperidone is the major active metabolite of risperidone, caution should be exercised when INVEGA TRINZA® is coadministered with risperidone or oral paliperidone for extended periods of time. Safety data involving concomitant use of INVEGA TRINZA® with other antipsychotics is limited.
Dosage Adjustment in Renal Impairment
INVEGA TRINZA® has not been systematically studied in patients with renal impairment [see Clinical Pharmacology (12.3)]. For patients with mild renal impairment (creatinine clearance ≥ 50 mL/min to < 80 mL/min [Cockcroft-Gault Formula], adjust dosage and stabilize the patient using the 1-month paliperidone palmitate extended-release injectable suspension, then transition to INVEGA TRINZA® [see Table 1, Dosage and Administration (2.2)]. [See also Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]
INVEGA TRINZA® is not recommended in patients with moderate or severe renal impairment (creatinine clearance < 50 mL/min) [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
Switching from INVEGA TRINZA® to the 1-Month Paliperidone Palmitate Extended-Release Injectable Suspension
For switching from INVEGA TRINZA® to INVEGA SUSTENNA® (1-month paliperidone palmitate extended-release injectable suspension), the 1-month paliperidone palmitate extended-release injectable suspension should be started 3 months after the last INVEGA TRINZA® dose, using the equivalent 3.5-fold lower dose as shown in Table 3. The 1-month paliperidone palmitate extended-release injectable suspension should then continue, dosed at monthly intervals.
| If the Last Dose of INVEGA TRINZA® is: | Initiate* INVEGA SUSTENNA® 3 Months Later at the Following Dose: |
|---|---|
|
|
| 273 mg | 78 mg |
| 410 mg | 117 mg |
| 546 mg | 156 mg |
| 819 mg | 234 mg |
Switching from INVEGA TRINZA® to Oral Paliperidone Extended-Release Tablets
For switching from INVEGA TRINZA® to oral paliperidone extended-release tablets, the daily dosing of the paliperidone extended-release tablets should be started 3 months after the last INVEGA TRINZA® dose and transitioned over the next several months following the last INVEGA TRINZA® dose as described in Table 4. Table 4 provides dose conversion regimens to allow patients previously stabilized on different doses of INVEGA TRINZA® to attain similar paliperidone exposure with once daily paliperidone extended-release tablets.
| Weeks Since Last INVEGA TRINZA® Dose | |||
|---|---|---|---|
| 3 months to 18 weeks | Longer than 18 weeks to 24 weeks | Longer than 24 weeks | |
| Last INVEGA TRINZA® Dose | Doses of oral paliperidone extended-release tablets | ||
| 273 mg | 3 mg | 3 mg | 3 mg |
| 410 mg | 3 mg | 3 mg | 6 mg |
| 546 mg | 3 mg | 6 mg | 9 mg |
| 819 mg | 6 mg | 9 mg | 12 mg |
Instructions for Use
 |
Administer every 3 months |
 |
Shake syringe vigorously for at least 15 seconds |
For intramuscular injection only. Do not administer by any other route.
Important
INVEGA TRINZA® should be administered by a healthcare professional as a single injection. DO NOT divide dose into multiple injections.
INVEGA TRINZA® is intended for intramuscular use only. Inject slowly, deep into the muscle taking care to avoid injection into a blood vessel.
Read complete instructions prior to use.
Dosing
This medication should be administered once every 3 months.
Preparation
Peel off tab label from the syringe and place in patient record.
INVEGA TRINZA® requires longer and more vigorous shaking than INVEGA SUSTENNA® (1-month paliperidone palmitate extended-release injectable suspension). Shake the syringe vigorously, with the syringe tip pointing up, for at least 15 seconds within 5 minutes prior to administration (see Step 2).
Thin Wall Safety Needle Selection
Thin wall safety needles are designed to be used with INVEGA TRINZA®. Therefore, it is important to only use the needles provided in the INVEGA TRINZA® kit.
Dose pack contents
| Prefilled Syringe | Thin Wall Safety Needles | ||||
|---|---|---|---|---|---|
 |
|||||
1 Select needle
Needle selection is determined by injection area and patient weight.
If administering a Deltoid injection If patient weighs: Less than 90 kg pink hub  |
If administering a Gluteal injection If patient weighs: Less than 90 kg yellow hub  |
| 90 kg or more yellow hub  |
90 kg or more yellow hub  |
 |
|

Check suspension
After shaking the syringe for at least 15 seconds, check the liquid in the viewing window.
The suspension should appear uniform and milky white in color.
It is also normal to see small air bubbles.
Open needle pouch and remove cap
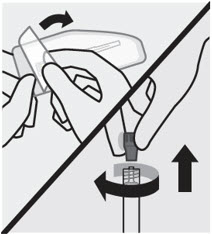
First, open needle pouch by peeling the cover back half way. Place on a clean surface.
Then, holding the syringe upright, twist and pull the rubber cap to remove.
Grasp needle pouch
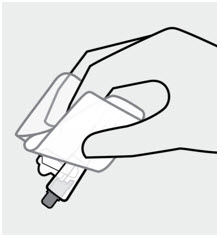
Fold back needle cover and plastic tray. Then, firmly grasp the needle sheath through the pouch, as shown.
Attach needle
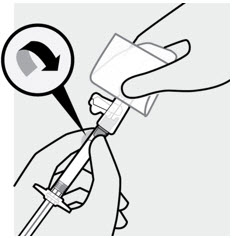
With your other hand, hold the syringe by the luer connection and attach it to the safety needle with a gentle clockwise twisting motion.
Do not remove the pouch until the syringe and needle are securely attached.
Remove needle sheath
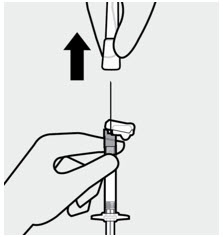
Pull the needle sheath away from the needle in a straight motion.
Do not twist the sheath, as this may loosen the needle from the syringe.
Remove air bubbles
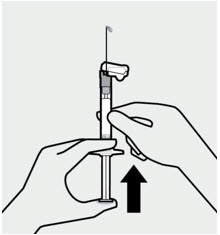
Hold the syringe upright and tap gently to make any air bubbles rise to the top.
Remove air by pressing the plunger rod upward carefully until a drop of liquid comes out of the needle tip.
3 Inject
Inject dose
Slowly inject the entire contents of the syringe intramuscularly, deep into the selected deltoid or gluteal muscle.
Do not administer by any other route.
4 After injection
Secure needle
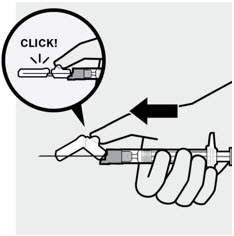
After the injection is complete, use your thumb or a flat surface to secure the needle in the safety device. The needle is secure when a "click" sound is heard.
Dispose properly
Dispose of the syringe and unused needle in an approved sharps container.





