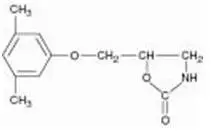
Drug Detail:Skelaxin (Metaxalone [ me-tax-a-lone ])
Drug Class: Skeletal muscle relaxants
Skelaxin - Clinical Pharmacology
Precautions
SKELAXIN should be administered with great care to patients with pre-existing liver damage. Serial liver function studies should be performed in these patients.
False-positive Benedict's tests, due to an unknown reducing substance, have been noted. A glucose-specific test will differentiate findings.
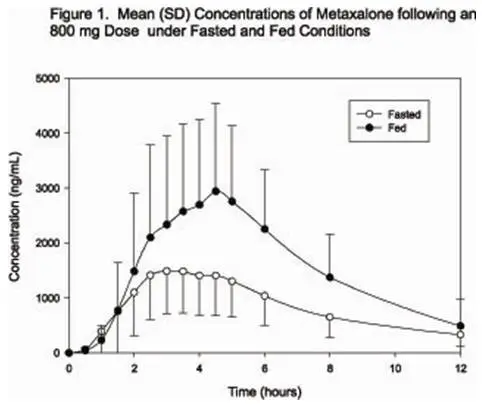
Taking SKELAXIN with food may enhance general CNS depression; elderly patients may be especially susceptible to this CNS effect (see CLINICAL PHARMACOLOGY: Pharmacokinetics and PRECAUTIONS: Information for Patients).
Overdosage
Deaths by deliberate or accidental overdose have occurred with metaxalone, particularly in combination with antidepressants, and have been reported with this class of drug in combination with alcohol.
Serotonin syndrome has been reported when SKELAXIN was used at doses higher than the recommended dose (see WARNINGS and ADVERSE REACTIONS).
When determining the LD50 in rats and mice, progressive sedation, hypnosis, and finally respiratory failure were noted as the dosage increased. In dogs, no LD50 could be determined as the higher doses produced an emetic action in 15 to 30 minutes.
| SKELAXIN
metaxalone tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) |