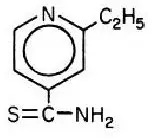
Drug Detail:Trecator (Ethionamide [ eth-eye-on-a-mide ])
Drug Class: Nicotinic acid derivatives
Indications and Usage for Trecator
Trecator is primarily indicated for the treatment of active tuberculosis in patients with M. tuberculosis resistant to isoniazid or rifampin, or when there is intolerance on the part of the patient to other drugs. Its use alone in the treatment of tuberculosis results in the rapid development of resistance. It is essential, therefore, to give a suitable companion drug or drugs, the choice being based on the results of susceptibility tests. If the susceptibility tests indicate that the patient's organism is resistant to one of the first-line anti-tuberculosis drugs (i.e., isoniazid or rifampin) yet susceptible to ethionamide, ethionamide should be accompanied by at least one drug to which the M. tuberculosis isolate is known to be susceptible.3 If the tuberculosis is resistant to both isoniazid and rifampin, yet susceptible to ethionamide, ethionamide should be accompanied by at least two other drugs to which the M. tuberculosis isolate is known to be susceptible.3
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Trecator and other antibacterial drugs, Trecator should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Patient nonadherence to prescribed treatment can result in treatment failure and in the development of drug-resistant tuberculosis, which can be life-threatening and lead to other serious health risks. It is, therefore, essential that patients adhere to the drug regimen for the full duration of treatment. Directly observed therapy is recommended for all patients receiving treatment for tuberculosis. Patients in whom drug-resistant M. tuberculosis organisms are isolated should be managed in consultation with an expert in the treatment of drug-resistant tuberculosis.
Warnings
The use of Trecator alone in the treatment of tuberculosis results in rapid development of resistance. It is essential, therefore, to give a suitable companion drug or drugs, the choice being based on the results of susceptibility testing. However, therapy may be initiated prior to receiving the results of susceptibility tests as deemed appropriate by the physician. Ethionamide should be administered with at least one, sometimes two, other drugs to which the organism is known to be susceptible (see INDICATIONS AND USAGE). Drugs which have been used as companion agents are rifampin, ethambutol, pyrazinamide, cycloserine, kanamycin, streptomycin, and isoniazid. The usual warnings, precautions, and dosage regimens for these companion drugs should be observed.
Patient compliance is essential to the success of the anti-tuberculosis therapy and to prevent the emergence of drug-resistant organisms. Therefore, patients should adhere to the drug regimen for the full duration of treatment. It is recommended that directly observed therapy be practiced when patients are receiving anti-tuberculous medication. Additional consultation from experts in the treatment of drug-resistant tuberculosis is recommended when patients develop drug-resistant organisms.
Precautions
Drug Interactions
Trecator has been found to temporarily raise serum concentrations of isoniazid. Trecator may potentiate the adverse effects of other anti-tuberculous drugs administered concomitantly. In particular, convulsions have been reported when ethionamide is administered with cycloserine and special care should be taken when the treatment regimen includes both of these drugs. Excessive ethanol ingestion should be avoided because a psychotic reaction has been reported.
Trecator Dosage and Administration
In the treatment of tuberculosis, a major cause of the emergence of drug-resistant organisms, and thus treatment failure, is patient nonadherence to prescribed treatment. Treatment failure and drug-resistant organisms can be life-threatening and may result in other serious health risks. It is, therefore, important that patients adhere to the drug regimen for the full duration of treatment. Directly observed therapy is recommended when patients are receiving treatment for tuberculosis. Consultation with an expert in the treatment of drug-resistant tuberculosis is advised for patients in whom drug-resistant tuberculosis is suspected or likely. Ethionamide should be administered with at least one, sometimes two, other drugs to which the organism is known to be susceptible (see INDICATIONS AND USAGE).
Trecator is administered orally. The usual adult dose is 15 to 20 mg/kg/day, administered once daily or, if patient exhibits poor gastrointestinal tolerance, in divided doses, with a maximum daily dosage of 1 gram.
Trecator tablets have been reformulated from a sugar-coated tablet to a film-coated tablet. Patients should be monitored and have their dosage retitrated when switching from the sugar-coated tablet to the film-coated tablet (see CLINICAL PHARMACOLOGY).
Therapy should be initiated at a dose of 250 mg daily, with gradual titration to optimal doses as tolerated by the patient. A regimen of 250 mg daily for 1 or 2 days, followed by 250 mg twice daily for 1 or 2 days with a subsequent increase to 1 gm in 3 or 4 divided doses has been reported.4,5 Thus far, there is insufficient evidence to indicate the lowest effective dosage levels. Therefore, in order to minimize the risk of resistance developing to the drug or to the companion drug, the principle of giving the highest tolerated dose (based on gastrointestinal intolerance) has been followed. In the adult this would seem to be between 0.5 and 1.0 gm daily, with an average of 0.75 gm daily.
The optimum dosage for pediatric patients has not been established. However, pediatric dosages of 10 to 20 mg/kg p.o. daily in 2 or 3 divided doses given after meals or 15 mg/kg/24 hrs as a single daily dose have been recommended.1,2 As with adults, ethionamide may be administered to pediatric patients once daily. It should be noted that in patients with concomitant tuberculosis and HIV infection, malabsorption syndrome may be present. Drug malabsorption should be suspected in patients who adhere to therapy, but who fail to respond appropriately. In such cases, consideration should be given to therapeutic drug monitoring (see CLINICAL PHARMACOLOGY).
The best times of administration are those which the individual patient finds most suitable in order to avoid or minimize gastrointestinal intolerance, which is usually at mealtimes. Every effort should be made to encourage patients to persevere with treatment when gastrointestinal side effects appear, since they may diminish in severity as treatment proceeds.
Concomitant administration of pyridoxine is recommended.
Duration of treatment should be based on individual clinical response. In general, continue therapy until bacteriological conversion has become permanent and maximal clinical improvement has occurred.
| TRECATOR
ethionamide tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc. (113008515) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Manufacturing Deutschland GmbH | 341970073 | ANALYSIS(0008-4117) , LABEL(0008-4117) , MANUFACTURE(0008-4117) , PACK(0008-4117) | |